Spatial Variation in Tree Density and Estimated Aboveground Carbon Stocks in Southern Africa
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Social Security in the Urban Fringe of Lilongwe City, Malawi
Shifting Boundaries: Social Security in the Urban Fringe of Lilongwe City, Malawi Wijkende grenzen: sociale zekerheid in de zelfkant van Lilongwe City, Malawi Proefschrift ter verkrijging van de graad van doctor aan de Erasmus Universiteit Rotterdam op gezag van de rector magnificus Prof. dr. S.W.J. Lamberts en volgens besluit van het College voor Promoties. De openbare verdediging zal plaatsvinden op donderdag 5 oktober 2006 om 16.00 uur door Barbara Anna Rohregger Geboren te Oberwart, Oostenreijk Promotiecomissie Promotor: Prof.dr. C.E. von Benda-Beckmann Overige leden: Prof. dr. N.J.H. Huls Prof. dr. W. van Binsbergen Dr. M.E. de Bruijn He re-enters Cape Town on the N2. He has been away less than three months, yet in that time the shanty settlements have crossed the highway and spread east of the airport. The stream of cars has to slow down while a child with a stick herds a stray cow off the road. Inexorably, he thinks, the country is coming to the city. Soon there will be cattle again on Rondebosch Common; soon history will have come full circle. (J.M. Coetzee, Disgrace) If you move, the support changes but your obligations remain the same. (Interview No. 107, Mr. Jameson) For Keebet who taught me much about my profession. For Matteo who taught me much about life. i Acknowledgements Writing about social networks also requires having a good one on one’s own. I would not have been able to write this book without the help of so many who have accompanied me during this process. -

Mozambique Zambia South Africa Zimbabwe Tanzania
UNITED NATIONS MOZAMBIQUE Geospatial 30°E 35°E 40°E L a k UNITED REPUBLIC OF 10°S e 10°S Chinsali M a l a w TANZANIA Palma i Mocimboa da Praia R ovuma Mueda ^! Lua Mecula pu la ZAMBIA L a Quissanga k e NIASSA N Metangula y CABO DELGADO a Chiconono DEM. REP. OF s a Ancuabe Pemba THE CONGO Lichinga Montepuez Marrupa Chipata MALAWI Maúa Lilongwe Namuno Namapa a ^! gw n Mandimba Memba a io u Vila úr L L Mecubúri Nacala Kabwe Gamito Cuamba Vila Ribáué MecontaMonapo Mossuril Fingoè FurancungoCoutinho ^! Nampula 15°S Vila ^! 15°S Lago de NAMPULA TETE Junqueiro ^! Lusaka ZumboCahora Bassa Murrupula Mogincual K Nametil o afu ezi Namarrói Erego e b Mágoè Tete GiléL am i Z Moatize Milange g Angoche Lugela o Z n l a h m a bez e i ZAMBEZIA Vila n azoe Changara da Moma n M a Lake Chemba Morrumbala Maganja Bindura Guro h Kariba Pebane C Namacurra e Chinhoyi Harare Vila Quelimane u ^! Fontes iq Marondera Mopeia Marromeu b am Inhaminga Velha oz P M úngu Chinde Be ni n è SOFALA t of ManicaChimoio o o o o o o o o o o o o o o o gh ZIMBABWE o Bi Mutare Sussundenga Dondo Gweru Masvingo Beira I NDI A N Bulawayo Chibabava 20°S 20°S Espungabera Nova OCE A N Mambone Gwanda MANICA e Sav Inhassôro Vilanculos Chicualacuala Mabote Mapai INHAMBANE Lim Massinga p o p GAZA o Morrumbene Homoíne Massingir Panda ^! National capital SOUTH Inhambane Administrative capital Polokwane Guijá Inharrime Town, village o Chibuto Major airport Magude MaciaManjacazeQuissico International boundary AFRICA Administrative boundary MAPUTO Xai-Xai 25°S Nelspruit Main road 25°S Moamba Manhiça Railway Pretoria MatolaMaputo ^! ^! 0 100 200km Mbabane^!Namaacha Boane 0 50 100mi !\ Bela Johannesburg Lobamba Vista ESWATINI Map No. -

AFRICA 40 20 Dublin 0 20 Minsk 40 60 IRE
AFRICA 40 20 Dublin 0 20 Minsk 40 60 IRE. U.K. Amsterdam Berlin London Warsaw BELARUS RUSSIA NETH. KAZAKHSTAN Brussels GERMANY POLAND Kiev BEL. LUX. Prague N o r t h CZ. REP. UKRAINE Vol Aral SLOV. ga Sea Paris Bratislava Rostov A t l a n t i c Vienna MOL. Chisinau SWITZ. Bern AUS. Budapest Tashkent HUNG. Sea of FRANCE SLO. ROM. Odesa Azov Ljubljana CRO. Belgrade 40 O c e a n Milan Zagreb Bucharest UZBEKISTAN Marseilles BOS. & Danube AND. HER. SER.& Black Sea GEO. Caspian ITALYSarajevo MONT. Sofia Tbilisi Sea Ponta BULG. TURKMENISTAN PORTUGAL Barcelona Corsica Istanbul AZER. Delgada Rome Skopje ARM. Baku Ashgabat AZORES Madrid Tirana MACE. Ankara Yerevan (PORTUGAL) Lisbon Naples ALB. SPAIN Sardinia GREECE . Mashhad Izmir TURKEY Tabriz- Adana Algiers Tunis Sicily Athens Tehran Strait of Gibraltar Oran Aleppo AFG. MADEIRA ISLANDS Constantine Valletta Nicosia (PORTUGAL) Rabat SYRIA IRAQ Fès MALTA LEB. Esfahan- Casablanca CYPRUS Damascus ¸ Funchal TUNISIA Mediterranean Sea Beirut IRAN MOROCCO Baghdad Jerusalem Amman - CANARY ISLANDS Marrakech Tripoli Banghazi- - Alexandria ISRAEL Shiraz (SPAIN) Bandar Cairo JORDAN Kuwait - KUWAIT 'Abbas Al Jizah- Persian Las Palmas Nile Laayoune A L G E R I A Manama Gulf (El Aaiún) Abu BAHR. Dhabi Western L I B Y A EGYPT Riyadh Doha Muscat Medina Sahara QATAR U.A.E Al Jawf Aswan- Tropic of OMAN Cancer Admin. SAUDI boundary Jiddah 20 Nouadhibou ARABIA 20 Mecca MAURITANIA S A H A R A Port Red Sudan Sea CAPE VERDE Nouakchott Nile Tombouctou N I G E R Praia Agadez Omdurman ERITREA YEMEN Dakar MALI Arabian SENEGAL Khartoum Asmara Sanaa Banjul er CHAD Nig Niamey Zinder Sea Bamako BURKINA Lac'Assal Gulf of THE GAMBIA S U D A N Blue FASO (lowest point in Socotra N'Djamena Africa, -155 m) Djibouti Aden Bissau Kano (YEMEN) Ouagadougou Nile DJIBOUTI GUINEA-BISSAU GUINEA Nile Conakry BENIN E Y NIGERIA L Hargeysa GHANA White Addis L Freetown Abuja Moundou A CÔTE Volta Ababa TOGO Ogbomoso V SIERRA LEONE D'IVOIRE ue Prov. -

Organized Crime and Instability in Central Africa
Organized Crime and Instability in Central Africa: A Threat Assessment Vienna International Centre, PO Box 500, 1400 Vienna, Austria Tel: +(43) (1) 26060-0, Fax: +(43) (1) 26060-5866, www.unodc.org OrgAnIzed CrIme And Instability In CenTrAl AFrica A Threat Assessment United Nations publication printed in Slovenia October 2011 – 750 October 2011 UNITED NATIONS OFFICE ON DRUGS AND CRIME Vienna Organized Crime and Instability in Central Africa A Threat Assessment Copyright © 2011, United Nations Office on Drugs and Crime (UNODC). Acknowledgements This study was undertaken by the UNODC Studies and Threat Analysis Section (STAS), Division for Policy Analysis and Public Affairs (DPA). Researchers Ted Leggett (lead researcher, STAS) Jenna Dawson (STAS) Alexander Yearsley (consultant) Graphic design, mapping support and desktop publishing Suzanne Kunnen (STAS) Kristina Kuttnig (STAS) Supervision Sandeep Chawla (Director, DPA) Thibault le Pichon (Chief, STAS) The preparation of this report would not have been possible without the data and information reported by governments to UNODC and other international organizations. UNODC is particularly thankful to govern- ment and law enforcement officials met in the Democratic Republic of the Congo, Rwanda and Uganda while undertaking research. Special thanks go to all the UNODC staff members - at headquarters and field offices - who reviewed various sections of this report. The research team also gratefully acknowledges the information, advice and comments provided by a range of officials and experts, including those from the United Nations Group of Experts on the Democratic Republic of the Congo, MONUSCO (including the UN Police and JMAC), IPIS, Small Arms Survey, Partnership Africa Canada, the Polé Institute, ITRI and many others. -

Crop Production Potential in South Africa's Neighboring P RSA 000/00/12510 Countries
DWA WATER RESOURCE STUDY IN SUPPORT OF THE ASGISA-EC MZIMVUBU DEVELOPMENT PROJECT LIST OF STUDY REPORTS REPORT DWA report number Summary Report P WMA 12/000/00/3609 Existing water supply infrastructure P WMA 12/000/00/3609 Volume 1 of 5 assessment Agricultural assessment and irrigation water P WMA 12/000/00/3609 Volume 2 of 5 use Groundwater assessment P WMA 12/000/00/3609 Volume 3 of 5 Water resources assessment P WMA 12/000/00/3609 Volume 4 of 5 Assessment of potential for pumped storage P WMA 12/000/00/3609 Volume 5 of 5 and hydropower schemes Rainwater Harvesting P WMA 12/000/00/3609 An assessment of rain-fed crop production potential in South Africa's neighboring P RSA 000/00/12510 countries AN ASSESSMENT OF RAIN-FED CROP PRODUCTION POTENTIAL IN SOUTH AFRICA'S NEIGHBORING COUNTRIES EXECUTIVE SUMMARY South Africa uses 60% of its scarce water resources on irrigation, a substantial portion of which is used to irrigate crops which are regarded internationally as rain-fed crops. The question is therefore being asked about the extent of alternative production areas in southern Africa (particularly in selected neighboring countries) for the range of crops which are presently produced sub-optimally under irrigation in South Africa. The objective of this study is therefore to provide an answer to this question with adequate confidence to allow the rational pursuit of this concept which could have far-reaching mutual benefit for southern African countries. The countries that were considered are Mozambique, Zimbabwe, Malawi and Zambia. -

Partnerships Program Coordinator Location
VACANCY Title: Partnerships Program Coordinator Location: Lilongwe, Malawi, Lagos, Nigeria or Cape Town, South Africa* *Only candidates who are legally authorized to work in one of these locations will Be considered To Apply: Please suBmit a CV and cover letter to [email protected] with “Partnerships Program Coordinator” on the suBject line. Only short-listed candidates will Be contacted. Overall Description: The Partnerships Program Coordinator will provide programmatic and administrative support across the Grassroot Soccer (GRS) Partnerships portfolio to ensure GRS and its partners are able to design, deliver and sustain high-quality programming, execute on grant awards, and steward strategic partnerships. Grassroot Soccer (GRS) is a rapidly growing adolescent health organization that leverages the power of soccer to educate, inspire, and mobilize at-risk youth in developing countries to overcome their greatest health challenges, live healthier, more productive lives, and be agents for change in their communities. Since 2002, GRS programs have reached 13 million young people in over 60 countries with life-saving HIV prevention and sexual and reproductive health information and services. Grassroot Soccer is looking to continue scaling its impact via partnerships over the next five years. Position Summary: The Partnerships Program Coordinator is a detail-oriented and dynamic team memBer who can leverage previous experience delivering and/or supporting adolescent health programming to strengthen GRS programs and partnerships. -

Global Suicide Rates and Climatic Temperature
SocArXiv Preprint: May 25, 2020 Global Suicide Rates and Climatic Temperature Yusuke Arima1* [email protected] Hideki Kikumoto2 [email protected] ABSTRACT Global suicide rates vary by country1, yet the cause of this variability has not yet been explained satisfactorily2,3. In this study, we analyzed averaged suicide rates4 and annual mean temperature in the early 21st century for 183 countries worldwide, and our results suggest that suicide rates vary with climatic temperature. The lowest suicide rates were found for countries with annual mean temperatures of approximately 20 °C. The correlation suicide rate and temperature is much stronger at lower temperatures than at higher temperatures. In the countries with higher temperature, high suicide rates appear with its temperature over about 25 °C. We also investigated the variation in suicide rates with climate based on the Köppen–Geiger climate classification5, and found suicide rates to be low in countries in dry zones regardless of annual mean temperature. Moreover, there were distinct trends in the suicide rates in island countries. Considering these complicating factors, a clear relationship between suicide rates and temperature is evident, for both hot and cold climate zones, in our dataset. Finally, low suicide rates are typically found in countries with annual mean temperatures within the established human thermal comfort range. This suggests that climatic temperature may affect suicide rates globally by effecting either hot or cold thermal stress on the human body. KEYWORDS Suicide rate, Climatic temperature, Human thermal comfort, Köppen–Geiger climate classification Affiliation: 1 Department of Architecture, Polytechnic University of Japan, Tokyo, Japan. -

Kampala Declaration on Community Paralegals
Kampala Declaration on Community Paralegals Kampala, Uganda July 26, 2012 Preamble We, the participants of a regional meeting on community paralegals held in Kampala on July 9- 11, 2012, recognize that in Africa and elsewhere the promises of law and government are often unmet. A health ministry pledges to treat tuberculosis, but many of its clinics have no drugs in stock; a mining firm uses its influence in the capital to override customary land rights; a fruit seller fails to obtain a trading license, because an official demands a bribe. Many people cannot avail themselves of nominally good rules and systems, because of cost, dysfunction, corruption, or abuse of power. In other cases, the law itself is unjust. As a result, many citizens are denied even basic rights to dignity, safety, and livelihood. We represent over fifty organizations working to advance justice in twenty African countries. Our collective experience has shown that community paralegals can help bridge the gap between law and society. Community paralegals use knowledge of law and government and tools like mediation, organizing, education, and advocacy to seek concrete solutions to instances of injustice. Community paralegals can straddle plural legal systems, engaging both formal and traditional institutions based on the needs of a given case. Paralegals are linked to lawyers who provide guidance and who can resort to litigation if frontline methods fail. Community paralegals have been active in Africa for decades, at least since the 1950s, when paralegals began assisting black South Africans to navigate and resist the codes of apartheid. Community paralegal efforts are diverse. -

Mozambique Atlas
FF II CC SS SS Field Information and Coordination Support Section Mozambique Division of Operational Services Sources: UNHCR, Global Insight digital mapping As of December 2009 © 1998 Europa Technologies Ltd. The boundaries and names shown and the designations used on this map do not imply official endorsement or acceptance by the United Nations. Mozambique_Atlas_A3PC.WOR KarongaKaronga KarongaKaronga ((( Lindi !! Kasama UNITED Nachingwea Mtwara (((!! ((( Songea REPUBLIC ((( Kitunguli !! !! !! !! !! ((( ((( ((( ((( Masasi ((( ((( Palma OF TANZANIA ((( Mbinga ((( ((( Newala !! Tunduru !! Vila de Mocímboa da Praia ((( Mzuzu ((( Mueda ((( MORONIMORONI ((( ((( ((( Mpika ((( ((( ((( Mzimba La ke Malawi ((( COMOROS ((( Ibo !! !! Nkhota Kota !! Porto Amelia MALAWI ((( ZAMBIA ((( Vila Cabral !! Chipata !! DzalekaDzaleka INDIAN OCEAN LILONGWELILONGWE ((( Nacala Velha Nova Freixo ((( ((( Mutuali ((( Lumbo (((!! Moçambique !! NampulaNampula !! Zomba MarrataneMarratane Blantyre !!!! Limbe MOZAMBIQUE ZIMBABWE Capital UNHCR Country Office HARAREHARARE !! Quelimane / National Office / Liaison Office ((( !!((( Eiffel Flats ((( Rusape UNHCR Field office !! !! UNHCR Field Unit !! Refugee camp !! Beira C Fort Victoria Refugee settlement !! CC Refugee transit centre !! Shabani CCTongogaraTongogara !! Main town or village ((( Secondary town or village (( Simplified entry point !! Ambo ¼¼ Official entry point ¼¼ !! ¼¼ !! ¼¼ !! Mozambique Channel !! Morombe Town of interest ((( MusinaMusina International boundary Main road !! Secondary -
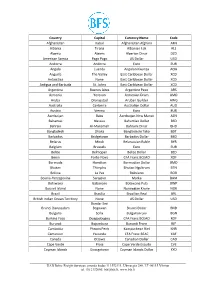
International Currency Codes
Country Capital Currency Name Code Afghanistan Kabul Afghanistan Afghani AFN Albania Tirana Albanian Lek ALL Algeria Algiers Algerian Dinar DZD American Samoa Pago Pago US Dollar USD Andorra Andorra Euro EUR Angola Luanda Angolan Kwanza AOA Anguilla The Valley East Caribbean Dollar XCD Antarctica None East Caribbean Dollar XCD Antigua and Barbuda St. Johns East Caribbean Dollar XCD Argentina Buenos Aires Argentine Peso ARS Armenia Yerevan Armenian Dram AMD Aruba Oranjestad Aruban Guilder AWG Australia Canberra Australian Dollar AUD Austria Vienna Euro EUR Azerbaijan Baku Azerbaijan New Manat AZN Bahamas Nassau Bahamian Dollar BSD Bahrain Al-Manamah Bahraini Dinar BHD Bangladesh Dhaka Bangladeshi Taka BDT Barbados Bridgetown Barbados Dollar BBD Belarus Minsk Belarussian Ruble BYR Belgium Brussels Euro EUR Belize Belmopan Belize Dollar BZD Benin Porto-Novo CFA Franc BCEAO XOF Bermuda Hamilton Bermudian Dollar BMD Bhutan Thimphu Bhutan Ngultrum BTN Bolivia La Paz Boliviano BOB Bosnia-Herzegovina Sarajevo Marka BAM Botswana Gaborone Botswana Pula BWP Bouvet Island None Norwegian Krone NOK Brazil Brasilia Brazilian Real BRL British Indian Ocean Territory None US Dollar USD Bandar Seri Brunei Darussalam Begawan Brunei Dollar BND Bulgaria Sofia Bulgarian Lev BGN Burkina Faso Ouagadougou CFA Franc BCEAO XOF Burundi Bujumbura Burundi Franc BIF Cambodia Phnom Penh Kampuchean Riel KHR Cameroon Yaounde CFA Franc BEAC XAF Canada Ottawa Canadian Dollar CAD Cape Verde Praia Cape Verde Escudo CVE Cayman Islands Georgetown Cayman Islands Dollar KYD _____________________________________________________________________________________________ -

Supplementary Material Barriers and Facilitators to Pre-Exposure
Sexual Health, 2021, 18, 130–39 © CSIRO 2021 https://doi.org/10.1071/SH20175_AC Supplementary Material Barriers and facilitators to pre-exposure prophylaxis among A frican migr ants in high income countries: a systematic review Chido MwatururaA,B,H, Michael TraegerC,D, Christopher LemohE, Mark StooveC,D, Brian PriceA, Alison CoelhoF, Masha MikolaF, Kathleen E. RyanA,D and Edwina WrightA,D,G ADepartment of Infectious Diseases, The Alfred and Central Clinical School, Monash Un iversity, Melbourne, Vic., Australia. BMelbourne Medical School, University of Melbourne, Melbourne, Vic., Australia. CSchool of Public Health and Preventative Medicine, Monash University, Melbourne, Vic., Australia. DBurnet Institute, Melbourne, Vic., Australia. EMonash Infectious Diseases, Monash Health, Melbourne, Vi, Auc. stralia. FCentre for Culture, Ethnicity & Health, Melbourne, Vic., Australia. GPeter Doherty Institute for Infection and Immunity, University of Melbourne, Melbourne, Vic., Australia. HCorresponding author. Email: [email protected] File S1 Appendix 1: Syntax Usedr Dat fo abase Searches Appendix 2: Table of Excluded Studies ( n=58) and Reasons for Exclusion Appendix 3: Critical Appraisal of Quantitative Studies Using the ‘ Joanna Briggs Institute Checklist for Analytical Cross-Sectional Studies’ (39) Appendix 4: Critical Appraisal of Qualitative Studies U sing a modified ‘CASP Qualitative C hecklist’ (37) Appendix 5: List of Abbreviations Sexual Health © CSIRO 2021 https://doi.org/10.1071/SH20175_AC Appendix 1: Syntax Used for Database -

Malawi Orientation Manual
Full Name of Republic of Malawi Country Population Malawi is home to roughly 19 million people. 84% of the population lives in rural areas. The life expectancy is 61 years, and the median age is 16.4 years (one of the lowest median ages in the world). Roughly 50.7% (2014 est.) live below the international poverty line. Time Zone GMT +2 (7 hours ahead of EST in the winter, 6 hours ahead in summer) Capital Lilongwe Ethnic Groups The African peoples in Malawi are all of Bantu origin. The main ethnic groups ('tribes') are the Chewa, dominant in the central and southern parts of the country; the Yao, also found in the south; and the Tumbuka in the north. There are very small populations of Asian (Indian, Pakistani, Korean and Chinese), white Africans and European people living mainly in the cities. Major Languages The official language of Malawi is Chichewa and English. English is widely spoken, particularly in main towns. The different ethnic groups in Malawi each have their own language or dialect. Major Religions Most people in Malawi are Christian (82.6%), usually members of one of the Catholic or Protestant churches founded by missionaries in the late 19th century. There are Muslims populations primarily in the south and central region (13%), especially along Lake Malawi - a legacy of the Arab slave traders who operated in this area. Alongside the established religions, many Malawians also hold traditional animist beliefs (2%). President’s Name In 2014, Peter Mutharika of the DPP followed his older brother Bingu wa Mutharika’s footsteps to become the current Malawian president.