Astrophysics with Radioactive Isotopes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nuclear Physics
Nuclear Physics Overview One of the enduring mysteries of the universe is the nature of matter—what are its basic constituents and how do they interact to form the properties we observe? The largest contribution by far to the mass of the visible matter we are familiar with comes from protons and heavier nuclei. The mission of the Nuclear Physics (NP) program is to discover, explore, and understand all forms of nuclear matter. Although the fundamental particles that compose nuclear matter—quarks and gluons—are themselves relatively well understood, exactly how they interact and combine to form the different types of matter observed in the universe today and during its evolution remains largely unknown. Nuclear physicists seek to understand not just the familiar forms of matter we see around us, but also exotic forms such as those that existed in the first moments after the Big Bang and that exist today inside neutron stars, and to understand why matter takes on the specific forms now observed in nature. Nuclear physics addresses three broad, yet tightly interrelated, scientific thrusts: Quantum Chromodynamics (QCD); Nuclei and Nuclear Astrophysics; and Fundamental Symmetries: . QCD seeks to develop a complete understanding of how the fundamental particles that compose nuclear matter, the quarks and gluons, assemble themselves into composite nuclear particles such as protons and neutrons, how nuclear forces arise between these composite particles that lead to nuclei, and how novel forms of bulk, strongly interacting matter behave, such as the quark-gluon plasma that formed in the early universe. Nuclei and Nuclear Astrophysics seeks to understand how protons and neutrons combine to form atomic nuclei, including some now being observed for the first time, and how these nuclei have arisen during the 13.8 billion years since the birth of the cosmos. -

The R-Process Nucleosynthesis and Related Challenges
EPJ Web of Conferences 165, 01025 (2017) DOI: 10.1051/epjconf/201716501025 NPA8 2017 The r-process nucleosynthesis and related challenges Stephane Goriely1,, Andreas Bauswein2, Hans-Thomas Janka3, Oliver Just4, and Else Pllumbi3 1Institut d’Astronomie et d’Astrophysique, Université Libre de Bruxelles, CP 226, 1050 Brussels, Belgium 2Heidelberger Institut fr¨ Theoretische Studien, Schloss-Wolfsbrunnenweg 35, 69118 Heidelberg, Germany 3Max-Planck-Institut für Astrophysik, Postfach 1317, 85741 Garching, Germany 4Astrophysical Big Bang Laboratory, RIKEN, 2-1 Hirosawa, Wako, Saitama, 351-0198, Japan Abstract. The rapid neutron-capture process, or r-process, is known to be of fundamental importance for explaining the origin of approximately half of the A > 60 stable nuclei observed in nature. Recently, special attention has been paid to neutron star (NS) mergers following the confirmation by hydrodynamic simulations that a non-negligible amount of matter can be ejected and by nucleosynthesis calculations combined with the predicted astrophysical event rate that such a site can account for the majority of r-material in our Galaxy. We show here that the combined contribution of both the dynamical (prompt) ejecta expelled during binary NS or NS-black hole (BH) mergers and the neutrino and viscously driven outflows generated during the post-merger remnant evolution of relic BH-torus systems can lead to the production of r-process elements from mass number A > 90 up to actinides. The corresponding abundance distribution is found to reproduce the∼ solar distribution extremely well. It can also account for the elemental distributions observed in low-metallicity stars. However, major uncertainties still affect our under- standing of the composition of the ejected matter. -

Status and Perspectives of the Neutron Time-Of-Flight Facility N TOF at CERN
Status and perspectives of the neutron time-of-flight facility n_TOF at CERN E. Chiaveri on behalf of the n_TOF Collaboration ([email protected]) Since the start of its operation in 2001, based on an idea of Prof. Carlo Rubbia[1], the neutron time-of-flight facility of CERN, n_TOF, has become one of the most forefront neutron facilities in the world for wide-energy spectrum neutron cross section measurements. Thanks to the combination of excellent neutron energy resolution and high instantaneous neutron flux available in the two experimental areas, the second of which has been constructed in 2014, n_TOF is providing a wealth of new data on neutron-induced reactions of interest for nuclear astrophysics, advanced nuclear technologies and medical applications. The unique features of the facility will continue to be exploited in the future, to perform challenging new measurements addressing the still open issues and long-standing quests in the field of neutron physics. In this document the main characteristics of the n_TOF facility and their relevance for neutron studies in the different areas of research will be outlined, addressing the possible future contribution of n_TOF in the fields of nuclear astrophysics, nuclear technologies and medical applications. In addition, the future perspectives of the facility will be described including the upgrade of the spallation target. 1 Introduction Neutron-induced reactions play a fundamental role for a number of research fields, from the origin of chemical elements in stars, to basic nuclear physics, to applications in advanced nuclear technology for energy, dosimetry, medicine and space science [1]. Thanks to the time-of-flight technique coupled with the characteristics of the CERN n_TOF beam-lines and neutron source, reaction cross-sections can be measured with a very high energy-resolution and in a broad neutron energy range from thermal up to GeV. -

White Paper on Nuclear Astrophysics and Low Energy Nuclear Physics
WHITE PAPER ON NUCLEAR ASTROPHYSICS AND LOW ENERGY NUCLEAR PHYSICS PART 1: NUCLEAR ASTROPHYSICS FEBRUARY 2016 NUCLEAR ASTROPHYSICS & LOW ENERGY NUCLEAR PHYSICS 1 Edited by: Hendrik Schatz and Michael Wiescher Layout and design: Erin O’Donnell, NSCL, Michigan State University Individual sections have been edited by the section conveners: Almudena Arcones, Dan Bardayan, Lee Bernstein, Jeffrey Blackmon, Edward Brown, Carl Brune, Art Champagne, Alessandro Chieffi, Aaron Couture, Roland Diehl, Jutta Escher, Brian Fields, Carla Froehlich, Falk Herwig, Raphael Hix, Christian Iliadis, Bill Lynch, Gail McLaughlin, Bronson Messer, Bradley Meyer, Filomena Nunes, Brian O'Shea, Madappa Prakash, Boris Pritychenko, Sanjay Reddy, Ernst Rehm, Grisha Rogachev, Bob Ruthledge, Michael Smith, Andrew Steiner, Tod Strohmayer, Frank Timmes, Remco Zegers, Mike Zingale NUCLEAR ASTROPHYSICS & LOW ENERGY NUCLEAR PHYSICS 2 ABSTRACT This white paper informs the nuclear astrophysics community and funding agencies about the scientific directions and priorities of the field and provides input from this community for the 2015 Nuclear Science Long Range Plan. It summarizes the outcome of the nuclear astrophysics town meeting that was held on August 21-23, 2014 in College Station at the campus of Texas A&M University in preparation of the NSAC Nuclear Science Long Range Plan. It also reflects the outcome of an earlier town meeting of the nuclear astrophysics community organized by the Joint Institute for Nuclear Astrophysics (JINA) on October 9- 10, 2012 Detroit, Michigan, with the purpose of developing a vision for nuclear astrophysics in light of the recent NRC decadal surveys in nuclear physics (NP2010) and astronomy (ASTRO2010). The white paper is furthermore informed by the town meeting of the Association of Research at University Nuclear Accelerators (ARUNA) that took place at the University of Notre Dame on June 12-13, 2014. -
R-Process: Observations, Theory, Experiment
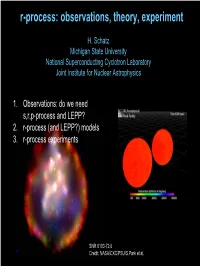
r-process: observations, theory, experiment H. Schatz Michigan State University National Superconducting Cyclotron Laboratory Joint Institute for Nuclear Astrophysics 1. Observations: do we need s,r,p-process and LEPP? 2. r-process (and LEPP?) models 3. r-process experiments SNR 0103-72.6 Credit: NASA/CXC/PSU/S.Park et al. Origin of the heavy elements in the solar system s-process: secondary • nuclei can be studied Æ reliable calculations • site identified • understood? Not quite … r-process: primary • most nuclei out of reach • site unknown p-process: secondary (except for νp-process) Æ Look for metal poor`stars (Pagel, Fig 6.8) To learn about the r-process Heavy elements in Metal Poor Halo Stars CS22892-052 (Sneden et al. 2003, Cowan) 2 1 + solar r CS 22892-052 ) H / X CS22892-052 ( g o red (K) giant oldl stars - formed before e located in halo Galaxyc was mixed n distance: 4.7 kpc theya preserve local d mass ~0.8 M_sol n pollutionu from individual b [Fe/H]= −3.0 nucleosynthesisa events [Dy/Fe]= +1.7 recall: element number[X/Y]=log(X/Y)-log(X/Y)solar What does it mean: for heavy r-process? For light r-process? • stellar abundances show r-process • process is not universal • process is universal • or second process exists (not visible in this star) Conclusions depend on s-process Look at residuals: Star – solar r Solar – s-process – p-process s-processSimmerer from Simmerer (Cowan et etal.) al. /Lodders (Cowan et al.) s-processTravaglio/Lodders from Travaglio et al. -0.50 -0.50 -1.00 -1.00 -1.50 -1.50 log e log e -2.00 -2.00 -2.50 -2.50 30 40 50 60 70 80 90 30 40 50 60 70 80 90 Element number Element number ÆÆNeedNeed reliable reliable s-process s-process (models (models and and nu nuclearclear data, data, incl. -
![Arxiv:1901.01410V3 [Astro-Ph.HE] 1 Feb 2021 Mental Information Is Available, and One Has to Rely Strongly on Theoretical Predictions for Nuclear Properties](https://docslib.b-cdn.net/cover/8159/arxiv-1901-01410v3-astro-ph-he-1-feb-2021-mental-information-is-available-and-one-has-to-rely-strongly-on-theoretical-predictions-for-nuclear-properties-508159.webp)
Arxiv:1901.01410V3 [Astro-Ph.HE] 1 Feb 2021 Mental Information Is Available, and One Has to Rely Strongly on Theoretical Predictions for Nuclear Properties
Origin of the heaviest elements: The rapid neutron-capture process John J. Cowan∗ HLD Department of Physics and Astronomy, University of Oklahoma, 440 W. Brooks St., Norman, OK 73019, USA Christopher Snedeny Department of Astronomy, University of Texas, 2515 Speedway, Austin, TX 78712-1205, USA James E. Lawlerz Physics Department, University of Wisconsin-Madison, 1150 University Avenue, Madison, WI 53706-1390, USA Ani Aprahamianx and Michael Wiescher{ Department of Physics and Joint Institute for Nuclear Astrophysics, University of Notre Dame, 225 Nieuwland Science Hall, Notre Dame, IN 46556, USA Karlheinz Langanke∗∗ GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany and Institut f¨urKernphysik (Theoriezentrum), Fachbereich Physik, Technische Universit¨atDarmstadt, Schlossgartenstraße 2, 64298 Darmstadt, Germany Gabriel Mart´ınez-Pinedoyy GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany; Institut f¨urKernphysik (Theoriezentrum), Fachbereich Physik, Technische Universit¨atDarmstadt, Schlossgartenstraße 2, 64298 Darmstadt, Germany; and Helmholtz Forschungsakademie Hessen f¨urFAIR, GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany Friedrich-Karl Thielemannzz Department of Physics, University of Basel, Klingelbergstrasse 82, 4056 Basel, Switzerland and GSI Helmholtzzentrum f¨urSchwerionenforschung, Planckstraße 1, 64291 Darmstadt, Germany (Dated: February 2, 2021) The production of about half of the heavy elements found in nature is assigned to a spe- cific astrophysical nucleosynthesis process: the rapid neutron capture process (r-process). Although this idea has been postulated more than six decades ago, the full understand- ing faces two types of uncertainties/open questions: (a) The nucleosynthesis path in the nuclear chart runs close to the neutron-drip line, where presently only limited experi- arXiv:1901.01410v3 [astro-ph.HE] 1 Feb 2021 mental information is available, and one has to rely strongly on theoretical predictions for nuclear properties. -

Investigations of Nuclear Decay Half-Lives Relevant to Nuclear Astrophysics
DE TTK 1949 Investigations of nuclear decay half-lives relevant to nuclear astrophysics PhD Thesis Egyetemi doktori (PhD) ´ertekez´es J´anos Farkas Supervisor / T´emavezet˝o Dr. Zsolt F¨ul¨op University of Debrecen PhD School in Physics Debreceni Egyetem Term´eszettudom´anyi Doktori Tan´acs Fizikai Tudom´anyok Doktori Iskol´aja Debrecen 2011 Prepared at the University of Debrecen PhD School in Physics and the Institute of Nuclear Research of the Hungarian Academy of Sciences (ATOMKI) K´esz¨ult a Debreceni Egyetem Fizikai Tudom´anyok Doktori Iskol´aj´anak magfizikai programja keret´eben a Magyar Tudom´anyos Akad´emia Atommagkutat´o Int´ezet´eben (ATOMKI) Ezen ´ertekez´est a Debreceni Egyetem Term´eszettudom´anyi Doktori Tan´acs Fizikai Tudom´anyok Doktori Iskol´aja magfizika programja keret´eben k´esz´ıtettem a Debreceni Egyetem term´eszettudom´anyi doktori (PhD) fokozat´anak elnyer´ese c´elj´ab´ol. Debrecen, 2011. Farkas J´anos Tan´us´ıtom, hogy Farkas J´anos doktorjel¨olt a 2010/11-es tan´evben a fent megnevezett doktori iskola magfizika programj´anak keret´eben ir´any´ıt´asommal v´egezte munk´aj´at. Az ´ertekez´esben foglalt eredm´e- nyekhez a jel¨olt ¨on´all´oalkot´otev´ekenys´eg´evel meghat´aroz´oan hozz´a- j´arult. Az ´ertekez´es elfogad´as´at javaslom. Debrecen, 2011. Dr. F¨ul¨op Zsolt t´emavezet˝o Investigations of nuclear decay half-lives relevant to nuclear astrophysics Ertekez´es´ a doktori (PhD) fokozat megszerz´ese ´erdek´eben a fizika tudom´any´agban ´Irta: Farkas J´anos, okleveles fizikus ´es programtervez˝omatematikus K´esz¨ult a Debreceni Egyetem Fizikai Tudom´anyok Doktori Iskol´aja magfizika programja keret´eben T´emavezet˝o: Dr. -

Low-Energy Nuclear Physics Part 2: Low-Energy Nuclear Physics
BNL-113453-2017-JA White paper on nuclear astrophysics and low-energy nuclear physics Part 2: Low-energy nuclear physics Mark A. Riley, Charlotte Elster, Joe Carlson, Michael P. Carpenter, Richard Casten, Paul Fallon, Alexandra Gade, Carl Gross, Gaute Hagen, Anna C. Hayes, Douglas W. Higinbotham, Calvin R. Howell, Charles J. Horowitz, Kate L. Jones, Filip G. Kondev, Suzanne Lapi, Augusto Macchiavelli, Elizabeth A. McCutchen, Joe Natowitz, Witold Nazarewicz, Thomas Papenbrock, Sanjay Reddy, Martin J. Savage, Guy Savard, Bradley M. Sherrill, Lee G. Sobotka, Mark A. Stoyer, M. Betty Tsang, Kai Vetter, Ingo Wiedenhoever, Alan H. Wuosmaa, Sherry Yennello Submitted to Progress in Particle and Nuclear Physics January 13, 2017 National Nuclear Data Center Brookhaven National Laboratory U.S. Department of Energy USDOE Office of Science (SC), Nuclear Physics (NP) (SC-26) Notice: This manuscript has been authored by employees of Brookhaven Science Associates, LLC under Contract No.DE-SC0012704 with the U.S. Department of Energy. The publisher by accepting the manuscript for publication acknowledges that the United States Government retains a non-exclusive, paid-up, irrevocable, world-wide license to publish or reproduce the published form of this manuscript, or allow others to do so, for United States Government purposes. DISCLAIMER This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, nor any of their contractors, subcontractors, or their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or any third party’s use or the results of such use of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. -

The U.S. Nuclear Reaction Data Network
BNL-NCS-63006 INFORMAL REPORT LIMITED DISTRIBUTION THE U.S. NUCLEAR REACTION DATA NETWORK Summary of the First Meeting held at the Colorado School of Mines Golden, Colorado March 13 & 14,1996 Assembled by M.R. Bhat National Nuclear Data Center Secretariat Department of Advanced Technology Brookhaven National Laboratory Associated Universities, Inc. Upton, Long Island, New York 11973 Under Contract No. DE-AC02-76CH00016 with the M A QTED United States Department of Energy *»! fl U I L P DISTRIBUTION OF THIS DOCUMENT IS UNLIMITED DISCLAIMER This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government nor any agency thereof, nor any of their employees, not any of their contractors, subcontractors, or their employees, makes any warranty, express or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name trademark, manufacturer, or otherwise, does not necessarily constitute or imply its endorsement, recommendation, or favoring by the United States Government or any agency, contractor, or subcontractor thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency, contractor or subcontractor thereof. ii TABLE OF CONTENTS Page 1. Agenda 1 2. Summary of the U.S. Nuclear Reaction Data Network Meeting M.R. Bhat, BNL 3 3. Attachment 1 Description of the U.S. -

Isotope Science Facility at Michigan State University: Nuclear Astrophysics
3. Nuclear astrophysics Nuclear reactions in stars and stellar explosions generate energy and are respon- 3 sible for the ongoing synthesis of the elements. They are, therefore, at the heart of many astrophysical phenomena, such as stars, novae, supernovae, and X-ray bursts. Nuclear physics determines the signatures of isotopic and elemental abun- dances found in the spectra of these objects, in characteristic γ radiation from nu- clear decay, or in the composition of meteorites and presolar grains. The field of nuclear astrophysics ties together nuclear and particle physics on the microscopic scale with the physics of stars, galaxies, and the cosmos in a broad interdisciplin- ary approach. While some of the major open questions of the field have been asked for decades, many others are new, mostly posed by advances in astronomical ob- servations. An example is the discovery of r-process abundance patterns in stars through high-resolution spectroscopic observations with ground-based observa- tories (for example Keck and VLT) as well as the Hubble Space Telescope. More of these stars are being discovered through extensive surveys, such as the Ham- burg/ESO R-process Enhanced Star Survey (HERES) or the ongoing Sloan Exten- sion for Galactic Understanding and Exploration (SEGUE). Superbursts, absorp- tion lines, and ms-oscillations are new phenomena discovered in X-ray bursts by the Chandra X-ray observatory, XMM-Newton, the Rossi X-ray Timing Explorer (RXTE), and Beppo-SAX. Many, if not most of the current open questions of the field require understanding of the physics of unstable nuclei. Though pioneering advances have been made, progress has been hampered by limited beam intensi- ties at current rare isotope beam facilities. -

Nuclear Reactions for Nuclear Astrophysics Weak Interactions and Fission in Stellar Nucleosynthesis
FACULTY OF SCIENCE UNIVERSITY OF AARHUS Nikolaj Thomas Zinner: Nuclear Reactions for Astrophysics Nuclear Reactions for Nuclear Astrophysics Weak Interactions and Fission in Stellar Nucleosynthesis Dissertation for the degree of Doctor of Philosophy Dissertation 2007 Nikolaj Thomas Zinner Department of Physics and Astronomy October 2007 Nuclear Reactions for Nuclear Astrophysics Weak Interactions and Fission in Stellar Nucleosynthesis Nikolaj Thomas Zinner Department of Physics and Astronomy University of Aarhus Dissertation for the degree of Doctor of Philosophy October 2007 @2007 Nikolaj Thomas Zinner 2nd Edition, October 2007 Department of Physics and Astronomy University of Aarhus Ny Munkegade, Bld. 1520 DK-8000 Aarhus C Denmark Phone: +45 8942 1111 Fax: +45 8612 0740 Email: [email protected] Cover Image: The evolution of the Universe from the Big Bang to the emer- gence of complex chemistry and Life. Printed by Reprocenter, Faculty of Science, University of Aarhus. This dissertation has been submitted to the Faculty of Science at the univer- sity of Aarhus in Denmark, in partial fulfillment of the requirements for the PhD degree in physics. The work presented has been performed under the supervision of Prof. Karlheinz Langanke. The work was mainly carried out at the Department of Physics and Astronomy in Aarhus. Numerous short- term visits to Gesellschaft f¨urSchwerionenforschung (GSI) in Darmstadt, Germany from 2005 to 2007 have been very fruitful toward the comple- tion of the thesis. The European Center for Theoretical Studies in Nuclear Physics and Related Areas (ECT*) in Trento, Italy is also acknowledged for its hospitality during the summer of 2004. There is something fascinating about science. -

Nuclear Astrophysics
Nuclear Astrophysics à Nuclear physics plays a special role in astronomy Nuclear structure: the DNA of chemical evolu;on (Woosley) Nuclear structure The composi;on of the solar system ) 6 Abundance (Si = 10 Element number (Z) The DNA of the cosmos Dillmann et al. 2003 Basic ques;ons in Nuclear Astrophysics: 1. What is the origin of the elements E0102 (SMC) - origin of elements in our solar composi;on SNR 0103-72.6 - Understanding composi;onal fingerprints of astrophysical events - Understanding composi;onal effects in stars, supernovae, neutron stars 2. How do stars and stellar explosions generate energy - Understand photon, neutrino emission - Understand how stars explode Supernova Ar;st’s view 3. What is the nature of neutron stars Neutron Star Ar;st’s view JINA a NSF Physics Fron;ers Center – www.jinaweb.org • Idenfy and address the crical open quesons and needs of the field • Form an intellectual center for the field • Overcome boundaries between astrophysics and nuclear physics and between theory and experiment • Aract and educate young people Nuclear Physics Experiments Astronomical Observations Astrophysical Models Associated: Nuclear Theory ANL, ASU, Princeton Core instuons: UCSB, UCSC, WMU • Notre Dame LANL, Victoria (Canada), • MSU EMMI (Germany), • U. of Chicago INPP Ohio, Minnesota Munich Cluster (Germany), http://www.jinaweb.org MoCA Monash (Australia) X-ray burst (RXTE) Supernova (Chandra,HST,..) 4U1728-34 331 Mass known 330 Half-life known nothing known 329 p process Frequency (Hz) 328 s-process E0102-72.3 327 10 15 20 Time