An Endophyte Constructs Fungicide-Containing Extracellular Barriers for Its Host Plant
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Finca+Slow+Permaculture.Pdf
Farming and Smallholding © Johanna McTiernan Dan McTiernan describes how regenerative agriculture is transforming olive groves in Spain and introduces © Johanna McTiernan transnational cropshare Restoring Agriculture in the Mediterranean “It’s not just that traditional Mediter- Together with our friends, who own healthy, perennial Mediterranean crops heavy input, bare-earth paradigm ranean agriculture isn’t sustainable a similar piece of land, and working that can’t be grown in Britain easily. of agriculture that is having such a ... it isn’t even viable on any level in partnership with IPM, we have If managed holistically, olives, nut destructive impact on the environ- anymore!” That was one of the first started Terra CSA, a multi-farm com- bearing trees such as almonds, and ment and the climate. All other things Richard Wade of Instituto munity supported agriculture project vine products like red wine, are about non-cold-pressed seed oils require Permacultura Montsant (IPM) said using permaculture and regenerative as perennial and sustainable as crops high levels of processing involving to us during our six month intern- agriculture to build soil and deliver come. We want the UK to still be heat and solvents in the extraction ship with him here in the south of olive oil, almonds and wine direct to able to access these incredibly process that are energy and resource Catalunya, Spain. cropshare members in the UK. nutritious products alongside the heavy and questionable in terms of With his doom laden words still Having been involved in community need to relocalise as much of our health to people and the planet. -
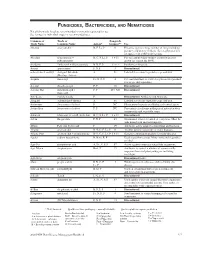
Fungicides, Bactericides, and Nematicides Not All Chemicals Listed Are Recommended Or Currently Registered for Use
FUNGICIDES, BACTERICIDES, AND NEMATICIDES Not all chemicals listed are recommended or currently registered for use. See listings for individual crops for recommended uses. Common or Trade or Fungicide Trade Name Common Name Action* Group #** Use Abound azoxystrobin B, F, Ls, P 11 Effective against a large number of fungi including powdery and downy mildews. Severe phytotoxicity on apples with a McIntosh heritage. Absolute tebuconazole + B, C, F, Ls, P 3 + 11 For rust and powdery mildew control in grasses trifloxystrobin grown for seed in the PNW. Academy fludioxonil + difenoconazole B-N, F, P 12 + 3 Postharvest fungicide. Accrue spiroxamine F, N, P 5 Discontinued. acibenzolar-S-methyl Actigard, Blockade A P1 Labeled for certain vegetable crops and fruit. (Heritage Action) Acquire metalaxyl Fs, N, P, S 4 For seed treatment to control ooymcetes in specified row crops and vegetables. Acrobat dimethomorph F, P 40 Discontinued. Acrobat MZ dimethomorph + F, P 40 + M3 Discontinued. mancozeb Acti-dione cycloheximide F Discontinued. Antibiotic and fungicide. Actigard acibenzolar-S-methyl A P1 Labeled for certain vegetable crops and fruit. Actinovate Streptomyces lydicus F NC Filamentous bacteria as a Biological control agent. Actino-Iron Streptomyces lydicus F, P NC For control of soilborne pathogens of indoor/outdoor ornamentals and vegetable crops. Adament tebuconazole + trifloxystrobin B, C, F, Ls, P 3 + 11 Discontinued. Adorn fluopicolide F, N, P 43 Ornamental label for control of oomycetes. Must be tank-mixed with another fungicide. Affirm Polyoxin D zinc salt F 19 Antibiotic active against certain fungi and bacteria. Aframe azoxystrobin B-N, C, F, Ls, P 11 Another generic fungicide for many diseases. -

A Sustainable Agricultural Future Relies on the Transition to Organic Agroecological Pest Management
sustainability Review A Sustainable Agricultural Future Relies on the Transition to Organic Agroecological Pest Management Lauren Brzozowski 1 and Michael Mazourek 1,2,* ID 1 Section of Plant Breeding and Genetics, School of Integrative Plant Science, Cornell University, Ithaca, NY 14853, USA; [email protected] 2 David R. Atkinson Center for Sustainable Future, Cornell University, Ithaca, NY 14853, USA * Correspondence: [email protected] Received: 21 April 2018; Accepted: 11 June 2018; Published: 15 June 2018 Abstract: The need to improve agricultural sustainability to secure yields, minimize environmental impacts and buffer environmental change is widely recognized. Investment in conventional agriculture has supported its present yield advantage. However, organic agriculture with agroecological management has nascent capacity for sustainable production and for increasing yields in the future. Conventional systems have leveraged reductionist approaches to address pests, primarily through pesticides that seek to eliminate biological factors that reduce yield, but come at a cost to human and ecosystem health, and leave production systems vulnerable to the development of pest resistance to these chemicals or traits. Alternatives are needed, and are found in organic production approaches. Although both organic and agroecology approaches encompass more than pest management, this aspect is a pivotal element of our agricultural future. Through increased investment and application of emerging analytical approaches to improve plant breeding for and management of these systems, yields and resilience will surpass approaches that address components alone. Keywords: organic agriculture; agroecology; pest management; plant breeding; biodiversity; sustainability; host plant resistance; pesticides 1. Achieving Needs for Agricultural Productivity and Pest Management Sustainably There is broad recognition among agricultural scientists that a growing world population will consume greater amounts of food and fiber with fewer resources available for production [1]. -

MP467 Arkansas Small Fruit Management Schedule 2021
DIVISION OF AGRICULTURE RESEARCH & EXTENSION MP467 University of Arkansas System Arkansas Small Fruit Management Schedule 2021 University of Arkansas, United States Department of Agriculture, and County Governments Cooperating Table of Contents Page Authors ......................................................................................................... 3 Disclaimer....................................................................................................... 4 Poison Control Center and Pesticide Spill Phone Numbers ................................................................ 4 Grapes – Commercial Growers ...................................................................................... 5 Grapes – Commercial Growers – Weed Control ......................................................................... 12 Blueberries – Commercial Growers ................................................................................... 15 Blueberries – Commercial Growers – Weed Control...................................................................... 18 Strawberries – Plasticulture – Commercial Growers ...................................................................... 21 Strawberries – Commercial Growers – Weed Control..................................................................... 24 Blackberries/Raspberries – Commercial Growers ........................................................................ 26 Blackberries/Raspberries – Commercial Growers – Weed Control.......................................................... -

Specimen Label • Long-Sleeved Shirt and Long Pants • Shoes Plus Socks
Handlers who may be exposed to the diluted product through application or other tasks must wear: Specimen Label • Long-sleeved shirt and long pants • Shoes plus socks. Handlers who may be exposed to the concentrate through mixing, loading, application, or other tasks must wear: • Coveralls over short-sleeved shirt and short pants • Chemical-resistant gloves such as butyl rubber, nitrile rubber, neoprene rubber or PVC • Chemical-resistant footwear plus socks • Protective eyewear • Chemical-resistant headgear for overhead exposure • Chemical-resistant apron when cleaning equipment, mixing or loading Discard clothing and other absorbent materials that have been drenched or heavily contaminated with this product’s concentrate. Do not reuse them. Follow manufacturer’s instructions for cleaning/maintaining PPE. If no such instructions for washables exist, use detergent and hot water. Keep and wash PPE separately from other laundry. User Safety Recommendations Users should: • Wash hands before eating, drinking, chewing gum, using tobacco, or For Commercial Use using the toilet. • Remove clothing/PPE immediately if pesticide gets inside. Then Active Ingredient wash thoroughly and put on clean clothing. Potassium salts of fatty acids.............................................. 049% Inert Ingredients.......................................................................... 051% Total............................................................................................ 100% First Aid If swallowed: Call a poison control center or doctor immediately for treatment advice. Have person sip a glass of water if able to swallow. Do not induce vomiting unless told to do so by the poison control center or doctor. Do not give anything by mouth to an unconscious person. Listed by the Organic Materials Review Institute (OMRI) for use in organic If on skin or clothing: Take off contaminated clothing. -

The Value of Fungicides in U.S. Crop Production
Crop Protection Research Institute The Value of Fungicides In U.S. Crop Production September 2005 Leonard P. Gianessi Nathan Reigner CropLife Foundation 1156 15th Street, NW #400 Washington, DC 20005 Phone 202-296-1585 www.croplifefoundation.org Fax 202-463-0474 This study was funded by CropLife America. The following organizations have reviewed the report’s case studies and have indicated their support for the findings. Almond Board of California American Sugarbeet Growers Association Artichoke Research Association California Asparagus Commission California Citrus Mutual California Citrus Quality Council California Dried Plum Board California Fresh Carrot Advisory Board California Grape and Tree Fruit League California Kiwifruit Commission California Minor Crops Council California Pepper Commission California Pistachio Commission California Strawberry Commission California Tree Fruit Agreement Cranberry Institute Cherry Marketing Institute Florida Farm Bureau Federation Georgia Fruit and Vegetable Association Georgia Pecan Growers Association Michigan Asparagus Advisory Board Michigan Onion Committee Michigan Potato Industry Commission Minnesota Cultivated Wild Rice Council Mint Industry Research Council National Association of Wheat Growers National Cotton Council National Onion Association National Potato Council North American Blueberry Council Oregon Hazelnut Commission Texas Citrus Mutual Texas Vegetable Association U.S. Apple Association U.S. Hop Industry Plant Protection Committee United Soybean Board Washington Red Raspberry Commission Washington Asparagus Commission Cover Photograph Credits Top Left: Fungicide crystal : Charles Krause, USDA, ARS Top Center & Right: Living, untreated and dead, treated spores: Dow AgroSciences Bottom Left: Fungicide protected potatoes: DuPont Crop Protection Bottom Right: Untreated, late blight infected potatoes: DuPont Crop Protection Table of Contents 1.0 Overview 2.0 Introduction A. Plant Diseases B. -

Bekah's Permaculture Assignment #7
1 1. In your own words what is “IPM”? IPM stands for integrated pest management. This is a term used to describe an approach that deals with pests (in gardens, fields, on plants, etc.). It is called integrative because it utilizes multiple approaches and techniques to deal with pest populations. This helps to make this approach more sustainable that traditional pesticides that the vast majority of people use. 2. Give 5 specific examples of IPM. There are several types of IPM. Here are just a few of them: 1) Increasing predator populations (such as attracting birds, bats, praying mantis, and other predatory creatures that help to control and reduce pest population) 2) Polyculture/crop combining (this means to plant a wide variety of things because certain pests tend to like certain plants and so the more variety there is, the more likely you will be able to control and limit pests) 3) Delaying (this means that you delay planting your crops/plants so that you avoid the peak season of the particular pest you are trying to avoid) 4) Planting flowers (this helps to attract predatory things such as bees and wasps and it can cause confusion amongst pests due to the color and smell of flowers) 5) Natural pesticides (this refers to making natural sprays, such as from crushed up pests, to spray on the plants to repel the pests) 3. Why don’t most people practice IPM? Most people don’t practice IPM because it takes more effort than simply spraying on pesticides. Also, many people don’t know about IPM and so they can’t practice what they don’t know. -

Antifungal Agents in Agriculture: Friends and Foes of Public Health
biomolecules Review Antifungal Agents in Agriculture: Friends and Foes of Public Health Veronica Soares Brauer 1, Caroline Patini Rezende 1, Andre Moreira Pessoni 1, Renato Graciano De Paula 2 , Kanchugarakoppal S. Rangappa 3, Siddaiah Chandra Nayaka 4, Vijai Kumar Gupta 5,* and Fausto Almeida 1,* 1 Department of Biochemistry and Immunology, Ribeirao Preto Medical School, University of Sao Paulo, Ribeirao Preto, SP 14049-900, Brazil; [email protected] (V.S.B.); [email protected] (C.P.R.); [email protected] (A.M.P.) 2 Department of Physiological Sciences, Health Sciences Centre, Federal University of Espirito Santo, Vitoria, ES 29047-105, Brazil; [email protected] 3 Department of Studies in Chemistry, University of Mysore, Manasagangotri, Mysore 570006, India; [email protected] 4 Department of Studies in Biotechnology, University of Mysore, Manasagangotri, Mysore 570006, India; [email protected] 5 Department of Chemistry and Biotechnology, ERA Chair of Green Chemistry, Tallinn University of Technology, 12618 Tallinn, Estonia * Correspondence: [email protected] (V.K.G.); [email protected] (F.A.) Received: 7 July 2019; Accepted: 19 September 2019; Published: 23 September 2019 Abstract: Fungal diseases have been underestimated worldwide but constitute a substantial threat to several plant and animal species as well as to public health. The increase in the global population has entailed an increase in the demand for agriculture in recent decades. Accordingly, there has been worldwide pressure to find means to improve the quality and productivity of agricultural crops. Antifungal agents have been widely used as an alternative for managing fungal diseases affecting several crops. However, the unregulated use of antifungals can jeopardize public health. -

2.5 Gallons Net Contents FIRST AID If on Skin Or • Take Off Contaminated Clothing
GROUP 1 4 HERBICIDES PULL HERE TO OPEN Postemergence herbicide for control of annual grass and broadleaf weeds in wheat and barley. Active Ingredient: Pinoxaden*: . 4.9% Fluroxypyr 1-methylheptyl ester**: . 12.4% Other Ingredients: 82.7% Total: 100.0% *CAS No. 243973-20-8 **CAS No. 81406-37-3 Contains 0.42 pounds of pinoxaden and 0.73 pounds of fluroxypyr acid equivalent per gallon. Contains petroleum distillates. KEEP OUT OF REACH OF CHILDREN. CAUTION See additional precautionary statements and directions for use inside booklet. EPA Reg. No. 100-1389 EPA Est. 100-NE-001 Product of United Kingdom Formulated in USA SCP 1389A-L1A 0112 4011309 2.5 gallons Net Contents FIRST AID If on skin or • Take off contaminated clothing. clothing • Rinse skin immediately with plenty of water for 15-20 minutes. • Call a poison control center or doctor for treatment advice. If in eyes • Hold eye open and rinse slowly and gently with water for 15-20 minutes. • Remove contact lenses, if present after the first 5 minutes, then continue rinsing eye. • Call a poison control center or doctor for treatment advice. If swallowed • Immediately call a poison control center or doctor. • Do not induce vomiting unless told to do so by a poison control center or doctor. • Do not give any liquid to the person. • Do not give anything by mouth to an unconscious person. If inhaled • Move person to fresh air. • If person is not breathing, call 911 or an ambulance, then give artificial respiration, prefer- ably by mouth-to-mouth, if possible. • Call a poison control center or doctor for further treatment advice. -

News Release
News Release BASF introduces Encartis, a new dual-active fungicide for the golf course market RESEARCH TRIANGLE PARK, NC, July 14, 2021 – BASF has introduced Encartis™ fungicide, a powerful dual-active fairway solution that provides preventative and curative activity against dollar spot and 10 other key foliar diseases. Encartis fungicide is offered in a new, easy-to-use, pre-mix formulation, combining the strength of both boscalid and chlorothalonil for strong preventive defense and fast curative action. The dual-active solution provides long lasting protection for up to 28 days to help maintain healthy, disease-free playing conditions all season long. “Encartis is an outstanding fungicide pre-mix and always performed very well in trials at Clemson,” said Dr. Bruce Martin, Turfgrass Pathology Professor Emeritus, Clemson University. “The combination extends the spectrum of diseases controlled while providing defense against selection of resistant fungal strains. I am glad to see this product added to turf managers’ tools for disease control.” While perfect for fairways, Encartis fungicide can be used anywhere on the golf course, including tees and greens, at any time of the year to control key diseases. Superintendents can learn more about Encartis fungicide by contacting their local BASF representative. Media Relations contact BASF Corporation Nicole Sanders 26 Davis Drive Phone: 704-560-5912 Research Triangle Park, NC [email protected] 27709 www.basf.com Always read and follow label directions. Encartis is a trademark of BASF. ®2021 BASF. All rights reserved. About BASF’s Agricultural Solutions division With a rapidly growing population, the world is increasingly dependent on our ability to develop and maintain sustainable agriculture and healthy environments. -

Potential Organic Fungicides for the Control of Powdery Mildew on Chrysanthemum X Morifolium
Potential Organic Fungicides for the Control of Powdery Mildew on Chrysanthemum x morifolium A Thesis Submitted in partial fulfillment of the requirements for the degree of Master of Science Michael Bradshaw University of Washington 2015 School of Environmental and Forest Science Thesis Committee Members Dr. Sarah Reichard (Committee Chair) Orin and Althea Soest Chair for Urban Horticulture Director, University of Washington Botanic Gardens Dr. Linda Chalker-Scott Associate Professor and Extension Urban Horticulturist Washington State University, PREC Dr. Marianne Elliott Research Associate, Plant Pathology Washington State University, PREC ©Copyright Michael Bradshaw Table of Contents ABSTRACT ............................................................................................................................... 1 INTRODUCTION ....................................................................................................................... 2 LITERATURE REVIEW ............................................................................................................. 4 Salts of Fatty Acids ............................................................................................................. 4 Organic Acids ..................................................................................................................... 6 Sesame Oil......................................................................................................................... 7 Inoculation Methods .......................................................................................................... -

Diplomacy of Fungicides: the Boon Becoming Threat for Sustainable Agriculture
Int.J.Curr.Microbiol.App.Sci (2019) 8(8): 1607-1612 International Journal of Current Microbiology and Applied Sciences ISSN: 2319-7706 Volume 8 Number 08 (2019) Journal homepage: http://www.ijcmas.com Review Article https://doi.org/10.20546/ijcmas.2019.808.189 A Review - Diplomacy of Fungicides: The Boon Becoming Threat for Sustainable Agriculture Rajesh Kumari1*, Govind Kumar Bagri3, Rahul Kumar Sharma1, Mohammad Akram1, Arshad Husain2 and Irsad1 1Department of Plant Protection, Faculty of Agricultural Sciences, Aligarh Muslim University, Aligarh, Uttar Pradesh-202002, India 2Department of Plant Pathology, Faculty of Agricultural Sciences, Chandra Shekhar Azad University of Agriculture and Technology, Kanpur-208002, India 3Department of Soil Science and Agricultural Chemistry, IAS, BHU, Varanasi-221005, India *Corresponding author ABSTRACT In order to feed increasing population there is need of solution which can provide quick result which is possible with fungicides. Fungicides are necessary evils to kill unwanted K e yw or ds pest and disease causing organisms in the agro-ecosystem. These are indispensible tool for protecting the crops and increasing food production. Soil applications of fungicides have Fungicides, been frequently used to control soil borne fungal diseases. Fungicides are used for killing Sustainable or inhibiting the growth of fungus. They are extensively used in pharmaceutical industry, Agriculture , agriculture, in protection of seed during storage and in preventing the growth of fungi that Fungicides produce toxins. Hence, fungicides production is constantly increasing as a result of their great importance to agriculture. On the other hand some fungicides affect humans and Article Info beneficial microorganisms including insects, birds and fish thus public concern about their Accepted: effects is increasing day by day.