Electrocommunication, Sensory Ecology, and Group Dynamics in a Mormyrid Weakly Electric Fish
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Functional Diversity of the Lateral Line System Among Populations of a Native Australian Freshwater Fish Lindsey Spiller1, Pauline F
© 2017. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2017) 220, 2265-2276 doi:10.1242/jeb.151530 RESEARCH ARTICLE Functional diversity of the lateral line system among populations of a native Australian freshwater fish Lindsey Spiller1, Pauline F. Grierson1, Peter M. Davies2, Jan Hemmi1,3, Shaun P. Collin1,3 and Jennifer L. Kelley1,* ABSTRACT Montgomery, 1999b; Bleckmann and Zelick, 2009; Montgomery Fishes use their mechanoreceptive lateral line system to sense et al., 1997). Correspondingly, ecological variables such as nearby objects by detecting slight fluctuations in hydrodynamic predation pressure (McHenry et al., 2009), habitat (Beckmann motion within their immediate environment. Species of fish from et al., 2010; Vanderpham et al., 2013) and water velocity (Wark and different habitats often display specialisations of the lateral line Peichel, 2010) may partly explain the diversity in lateral line system, in particular the distribution and abundance of neuromasts, morphology that is often observed in species occupying different but the lateral line can also exhibit considerable diversity within a habitats. species. Here, we provide the first investigation of the lateral line The functional link between lateral line morphology, habitat system of the Australian western rainbowfish (Melanotaenia variation and behaviour remains very poorly understood. For australis), a species that occupies a diversity of freshwater habitats example, while it is clear that the lateral line is used by larval across semi-arid northwest Australia. We collected 155 individuals zebrafish to respond to suction-feeding predators (McHenry et al., from eight populations and surveyed each habitat for environmental 2009), only one study has shown that exposure to environmental factors that may contribute to lateral line specialisation, including cues, such as predation risk, can affect the development of the lateral water flow, predation risk, habitat structure and prey availability. -

§4-71-6.5 LIST of CONDITIONALLY APPROVED ANIMALS November
§4-71-6.5 LIST OF CONDITIONALLY APPROVED ANIMALS November 28, 2006 SCIENTIFIC NAME COMMON NAME INVERTEBRATES PHYLUM Annelida CLASS Oligochaeta ORDER Plesiopora FAMILY Tubificidae Tubifex (all species in genus) worm, tubifex PHYLUM Arthropoda CLASS Crustacea ORDER Anostraca FAMILY Artemiidae Artemia (all species in genus) shrimp, brine ORDER Cladocera FAMILY Daphnidae Daphnia (all species in genus) flea, water ORDER Decapoda FAMILY Atelecyclidae Erimacrus isenbeckii crab, horsehair FAMILY Cancridae Cancer antennarius crab, California rock Cancer anthonyi crab, yellowstone Cancer borealis crab, Jonah Cancer magister crab, dungeness Cancer productus crab, rock (red) FAMILY Geryonidae Geryon affinis crab, golden FAMILY Lithodidae Paralithodes camtschatica crab, Alaskan king FAMILY Majidae Chionocetes bairdi crab, snow Chionocetes opilio crab, snow 1 CONDITIONAL ANIMAL LIST §4-71-6.5 SCIENTIFIC NAME COMMON NAME Chionocetes tanneri crab, snow FAMILY Nephropidae Homarus (all species in genus) lobster, true FAMILY Palaemonidae Macrobrachium lar shrimp, freshwater Macrobrachium rosenbergi prawn, giant long-legged FAMILY Palinuridae Jasus (all species in genus) crayfish, saltwater; lobster Panulirus argus lobster, Atlantic spiny Panulirus longipes femoristriga crayfish, saltwater Panulirus pencillatus lobster, spiny FAMILY Portunidae Callinectes sapidus crab, blue Scylla serrata crab, Samoan; serrate, swimming FAMILY Raninidae Ranina ranina crab, spanner; red frog, Hawaiian CLASS Insecta ORDER Coleoptera FAMILY Tenebrionidae Tenebrio molitor mealworm, -

Assessment of Coastal Water Resources and Watershed Conditions at Katmai National Park and Preserve (Alaska)
National Park Service U.S. Department of the Interior Natural Resources Program Center Assessment of Coastal Water Resources and Watershed Conditions at Katmai National Park and Preserve (Alaska) Natural Resource Technical Report NPS/NRWRD/NRTR—2007/372 Cover photo: Glacier emerging from the slopes of Mt Douglas toward the Katmai coastline. August 2005. Photo: S.Nagorski 2 Assessment of Coastal Water Resources and Watershed Conditions at Katmai National Park and Preserve (Alaska) Natural Resource Technical Report NPS/NRWRD/NRTR-2007/372 Sonia Nagorski Environmental Science Program University of Alaska Southeast Juneau, AK 99801 Ginny Eckert Biology Program University of Alaska Southeast Juneau, AK 99801 Eran Hood Environmental Science Program University of Alaska Southeast Juneau, AK 99801 Sanjay Pyare Environmental Science Program University of Alaska Southeast Juneau, AK 99801 This report was prepared under Task Order J9W88050014 of the Pacific Northwest Cooperative Ecosystem Studies Unit (agreement CA90880008) Water Resources Division Natural Resource Program Center 1201 Oakridge Drive, Suite 250 Fort Collins, CO 80525 June 2007 U.S. Department of Interior Washington, D.C. 3 The Natural Resource Publication series addresses natural resource topics that are of interest and applicability to a broad readership in the National Park Service and to others in the management of natural resources, including the scientific community, the public, and the NPS conservation and environmental constituencies. Manuscripts are peer-reviewed to ensure that the information is scientifically credible, technically accurate, appropriately written for the audience, and is designed and published in a professional manner. The Natural Resource Technical Reports series is used to disseminate the peer-reviewed results of scientific studies in the physical, biological, and social sciences for both the advancement of science and the achievement of the National Park Service’s mission. -

REVIEW Electric Fish: New Insights Into Conserved Processes of Adult Tissue Regeneration
2478 The Journal of Experimental Biology 216, 2478-2486 © 2013. Published by The Company of Biologists Ltd doi:10.1242/jeb.082396 REVIEW Electric fish: new insights into conserved processes of adult tissue regeneration Graciela A. Unguez Department of Biology, New Mexico State University, Las Cruces, NM 88003, USA [email protected] Summary Biology is replete with examples of regeneration, the process that allows animals to replace or repair cells, tissues and organs. As on land, vertebrates in aquatic environments experience the occurrence of injury with varying frequency and to different degrees. Studies demonstrate that ray-finned fishes possess a very high capacity to regenerate different tissues and organs when they are adults. Among fishes that exhibit robust regenerative capacities are the neotropical electric fishes of South America (Teleostei: Gymnotiformes). Specifically, adult gymnotiform electric fishes can regenerate injured brain and spinal cord tissues and restore amputated body parts repeatedly. We have begun to identify some aspects of the cellular and molecular mechanisms of tail regeneration in the weakly electric fish Sternopygus macrurus (long-tailed knifefish) with a focus on regeneration of skeletal muscle and the muscle-derived electric organ. Application of in vivo microinjection techniques and generation of myogenic stem cell markers are beginning to overcome some of the challenges owing to the limitations of working with non-genetic animal models with extensive regenerative capacity. This review highlights some aspects of tail regeneration in S. macrurus and discusses the advantages of using gymnotiform electric fishes to investigate the cellular and molecular mechanisms that produce new cells during regeneration in adult vertebrates. -
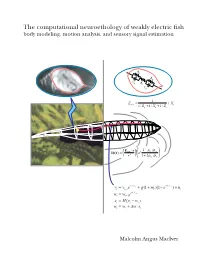
M.A. Maciver's Dissertation
The computational neuroethology of weakly electric fish body modeling, motion analysis, and sensory signal estimation R s R s C R s i C s 1 Z = + X prey + + s 1/ Rp 1/ Xp 1/ Zs −ρ ρ E ⋅r 1 p / w δφ(r) = fish a3 3 + ρ ρ r 1 2 p / w − t − t = 1/ v + + − 1/ v + vt vt−1e g(1 mt )(1 e ) nt − t = 1/ w wt wt−1e = − st H(vt wt ) = + ∆ ⋅ wt wt w st Malcolm Angus MacIver THE COMPUTATIONAL NEUROETHOLOGY OF WEAKLY ELECTRIC FISH: BODY MODELING, MOTION ANALYSIS, AND SENSORY SIGNAL ESTIMATION BY MALCOLM ANGUS MACIVER B.Sc., University of Toronto, 1991 M.A., University of Toronto, 1992 THESIS Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Neuroscience in the Graduate College of the University of Illinois at Urbana-Champaign, 2001 Urbana, Illinois c Copyright by Malcolm Angus MacIver, 2001 ABSTRACT Animals actively influence the content and quality of sensory information they acquire through the positioning of peripheral sensory surfaces. Investigation of how the body and brain work together for sensory acquisition is hindered by 1) the limited number of techniques for tracking sensory surfaces, few of which provide data on the position of the entire body surface, and by 2) our inability to measure the thousands of sensory afferents stimulated during behav- ior. I present research on sensory acquisition in weakly electric fish of the genus Apteronotus, where I overcame the first barrier by developing a markerless tracking system and have de- ployed a computational approach toward overcoming the second barrier. -

The Cyphomyrus Myers 1960 (Osteoglossiformes: Mormyridae) of the Lufira Basin (Upper Lualaba: DR Congo): a Generic Reassignment and the Description of a New Species
Received: 9 January 2019 Accepted: 15 December 2019 DOI: 10.1111/jfb.14237 SPECIAL ISSUE REGULAR PAPER FISH The Cyphomyrus Myers 1960 (Osteoglossiformes: Mormyridae) of the Lufira basin (Upper Lualaba: DR Congo): A generic reassignment and the description of a new species Christian Mukweze Mulelenu1,2,3,4 | Bauchet Katemo Manda2,3,4 | Eva Decru3,4 | Auguste Chocha Manda2 | Emmanuel Vreven3,4 1Département de Zootechnie, Faculté des Sciences Agronomiques, Université de Abstract Kolwezi, Kolwezi, Democratic Republic of the Within a comparative morphological framework, Hippopotamyrus aelsbroecki, only Congo known from the holotype originating from Lubumbashi, most probably the Lubumbashi 2Département de Gestion des Ressources Naturelles Renouvelables, Unité de recherche River, a left bank subaffluent of the Luapula River, is reallocated to the genus en Biodiversité et Exploitation durable des Cyphomyrus. This transfer is motivated by the fact that H. aelsbroecki possesses a Zones Humides, Université de Lubumbashi, Lubumbashi, Democratic Republic of the rounded or vaulted predorsal profile, an insertion of the dorsal fin far anterior to the Congo level of the insertion of the anal fin, and a compact, laterally compressed and deep 3Vertebrate Section, Ichthyology, Royal Museum for Central Africa, Tervuren, Belgium body. In addition, a new species of Cyphomyrus is described from the Lufira basin, 4Laboratory of Biodiversity and Evolutionary Cyphomyrus lufirae. Cyphomyrus lufirae was collected in large parts of the Middle Lufira, Genomics, KU Leuven, Leuven, Belgium upstream of the Kyubo Falls and just downstream of these falls in the lower Lufira and Correspondence its nearby left bank affluent, the Luvilombo River. The new species is distinguished Emmanuel Vreven, Curator of Fishes from all its congeners, that is, firstly, from C. -

Opinion Why Do Fish School?
Current Zoology 58 (1): 116128, 2012 Opinion Why do fish school? Matz LARSSON1, 2* 1 The Cardiology Clinic, Örebro University Hospital, SE -701 85 Örebro, Sweden 2 The Institute of Environmental Medicine, Karolinska Institute, SE-171 77 Stockholm, Sweden Abstract Synchronized movements (schooling) emit complex and overlapping sound and pressure curves that might confuse the inner ear and lateral line organ (LLO) of a predator. Moreover, prey-fish moving close to each other may blur the elec- tro-sensory perception of predators. The aim of this review is to explore mechanisms associated with synchronous swimming that may have contributed to increased adaptation and as a consequence may have influenced the evolution of schooling. The evolu- tionary development of the inner ear and the LLO increased the capacity to detect potential prey, possibly leading to an increased potential for cannibalism in the shoal, but also helped small fish to avoid joining larger fish, resulting in size homogeneity and, accordingly, an increased capacity for moving in synchrony. Water-movements and incidental sound produced as by-product of locomotion (ISOL) may provide fish with potentially useful information during swimming, such as neighbour body-size, speed, and location. When many fish move close to one another ISOL will be energetic and complex. Quiet intervals will be few. Fish moving in synchrony will have the capacity to discontinue movements simultaneously, providing relatively quiet intervals to al- low the reception of potentially critical environmental signals. Besides, synchronized movements may facilitate auditory grouping of ISOL. Turning preference bias, well-functioning sense organs, good health, and skillful motor performance might be important to achieving an appropriate distance to school neighbors and aid the individual fish in reducing time spent in the comparatively less safe school periphery. -

Order GASTEROSTEIFORMES PEGASIDAE Eurypegasus Draconis
click for previous page 2262 Bony Fishes Order GASTEROSTEIFORMES PEGASIDAE Seamoths (seadragons) by T.W. Pietsch and W.A. Palsson iagnostic characters: Small fishes (to 18 cm total length); body depressed, completely encased in Dfused dermal plates; tail encircled by 8 to 14 laterally articulating, or fused, bony rings. Nasal bones elongate, fused, forming a rostrum; mouth inferior. Gill opening restricted to a small hole on dorsolat- eral surface behind head. Spinous dorsal fin absent; soft dorsal and anal fins each with 5 rays, placed posteriorly on body. Caudal fin with 8 unbranched rays. Pectoral fins large, wing-like, inserted horizon- tally, composed of 9 to 19 unbranched, soft or spinous-soft rays; pectoral-fin rays interconnected by broad, transparent membranes. Pelvic fins thoracic, tentacle-like,withI spine and 2 or 3 unbranched soft rays. Colour: in life highly variable, apparently capable of rapid colour change to match substrata; head and body light to dark brown, olive-brown, reddish brown, or almost black, with dorsal and lateral surfaces usually darker than ventral surface; dorsal and lateral body surface often with fine, dark brown reticulations or mottled lines, sometimes with irregular white or yellow blotches; tail rings often encircled with dark brown bands; pectoral fins with broad white outer margin and small brown spots forming irregular, longitudinal bands; unpaired fins with small brown spots in irregular rows. dorsal view lateral view Habitat, biology, and fisheries: Benthic, found on sand, gravel, shell-rubble, or muddy bottoms. Collected incidentally by seine, trawl, dredge, or shrimp nets; postlarvae have been taken at surface lights at night. -

Chirping and Asymmetric Jamming Avoidance Responses in the Electric Fish Distocyclus Conirostris Jacquelyn M
© 2018. Published by The Company of Biologists Ltd | Journal of Experimental Biology (2018) 221, jeb178913. doi:10.1242/jeb.178913 SHORT COMMUNICATION Chirping and asymmetric jamming avoidance responses in the electric fish Distocyclus conirostris Jacquelyn M. Petzold1,2, JoséA. Alves-Gomes3 and G. Troy Smith1,2,* ABSTRACT of two of more EODs creates a periodic amplitude modulation Electrosensory systems of weakly electric fish must accommodate (beat). Beat frequency is equal to the difference between the EOD competing demands of sensing the environment (electrolocation) frequencies (EODfs) of the two interacting fish. Fish use the beat and receiving social information (electrocommunication). The and the relative geometry of the interacting signals to estimate jamming avoidance response (JAR) is a behavioral strategy thought conspecific EODfs, which convey important social information to reduce electrosensory interference from conspecific signals close (Smith, 2013; Dunlap, et al., 2017). However, slow beats (<10 Hz) in frequency. We used playback experiments to characterize electric created by interactions between fish with similar EODfs can impair organ discharge frequency (EODf), chirping behavior and the JAR of the electrolocation function of the EOD by masking localized EOD Distocyclus conirostris, a gregarious electric fish species. EODs of D. distortions (Heiligenberg, 1973; Matsubara and Heiligenberg, conirostris had low frequencies (∼80–200 Hz) that shifted in response 1978). The JAR is a stereotyped response in which an electric to playback stimuli. Fish consistently lowered EODf in response to fish increases or decreases its EODf to increase beat frequency and higher-frequency stimuli but inconsistently raised or lowered EODf in thereby reduce or eliminate the interference caused by slow beats response to lower-frequency stimuli. -

Hormones and Sexual Behavior of Teleost Fishes
Chapter 7 Hormones and Sexual Behavior of Teleost Fishes y David M. Gonc¸alves*, and Rui F. Oliveira*,** y * Instituto Superior de Psicologia Aplicada, Lisboa, Portugal, Universidade do Algarve, Faro, Portugal, ** Instituto Gulbenkian de Cieˆncia, Oeiras, Portugal more variable during the initial stages of the sequence and SUMMARY more stereotyped towards its end. To account for this Fishes are an excellent group for studying the mechanisms through which hormones modulate the expression of sexual variation, these researchers suggested that an initial appe- behaviors in vertebrates. First, they have radiated virtually titive phase, defined as the phase of searching towards the throughout all aquatic environments and this is reflected in an goal, can be distinguished from a final consummatory extraordinary diversity of mating systems and reproductive phase, defined as the stage when the goal is reached behaviors. Second, many species present a remarkable plasticity (Sherrington, 1906; Craig, 1917). Although this distinction in their sexual displays, as exemplified by fishes that change sex or is still widely applied in studies investigating the mecha- that adopt more than one reproductive tactic during their lifetime, nisms of behavior, there is an ongoing debate on the and this plasticity seems to be mediated by hormones. Third, the usefulness of these terms. In a recent review, Sachs (2007) fish neuroendocrine system is well conserved among vertebrates identified some problems in the current use of the and the mechanisms of hormonal action in behavior are likely to appetitive/consummatory dichotomy. These include the share similarities with those of other vertebrates. We review the difficulties in defining the boundary between the two pha- role of hormones and neuropeptides in the modulation of fish sexual displays. -

The Air-Breathing Behaviour of Brevimyrus Niger (Osteoglossomorpha, Mormyridae)
Journal of Fish Biology (2007) 71, 279–283 doi:10.1111/j.1095-8649.2007.01473.x, available onlineathttp://www.blackwell-synergy.com The air-breathing behaviour of Brevimyrus niger (Osteoglossomorpha, Mormyridae) T. MORITZ* AND K. E. LINSENMAIR Lehrstuhl fur¨ Tiero¨kologie und Tropenbiologie, Theodor-Boveri-Institut, Universita¨t Wurzburg,¨ Am Hubland, 97074 Wurzburg,¨ Germany (Received 2 May 2006, Accepted 6 February 2007) Brevimyrus niger is reported to breathe atmospheric air, confirming previous documenta- tion of air breathing in this species. Air is taken up by rising to the water surface and gulping, or permanently resting just below the surface, depending on the environmental conditions. # 2007 The Authors Journal compilation # 2007 The Fisheries Society of the British Isles Key words: elephantfishes; Osteoglossomorpha; weakly electric fish. The Mormyridae consists of 201 weakly electric fishes endemic to Africa (Nelson, 2006). They belong to the Osteoglossomorpha among which air-breathing behaviour is known from several families. All genera of the Osteoglossidae are able to breathe atmospheric air utilizing their swimbladder as a respiratory organ, i.e. Heterotis niloticus (Cuvier) (Luling,¨ 1977), Arapaima gigas (Schinz) (Luling,¨ 1964, 1977). Similarly, Pantodon buchholzi Peters (Schwarz, 1969), which is the only member of the Pantodontidae, and the members of the Notopteridae (Graham, 1997) are air-breathers. A close relative to the mormyrids, Gymnarchus niloticus Cuvier, the only member of the Gymnarchidae, is also well known to breathe air (Hyrtl, 1856; Bertyl, 1958). In the remaining two families within the Osteoglossomorpha air breathing has never been reported from the Hiodontidae (Graham, 1997) and only for a single species, Brevimyrus niger (Gunther),¨ within the Mormyridae (Benech & Lek, 1981; Bigorne, 2003). -

JAGE-691 Fish Cognition and Consciousness Colin Allen [email protected] Phone
JAGE-691 Fish Cognition and Consciousness Colin Allen [email protected] phone: +1-812-606-0881 fax: +1-812-855-3631 Program in Cognitive Science and Department of History and Philosophy of Science Indiana University, Bloomington, IN 47405 USA ABSTRACT. Questions about fish consciousness and cognition are receiving increasing attention. In this paper, I explain why one must be careful to avoid drawing conclusions too hastily about this hugely di- verse set of species. Keywords. Fish, learning, cognition, consciousness 1. Introduction to the controversy The cognitive and mental capacities of fish are a current topic of scientific controversy, and consciousness is the most contentious of topics. In a recent review article, Michel Cabanac and coauthors (Cabanac et al. 2009) argue that consciousness did not emerge until the early Amniota, the group of species that includes mammals, birds, and "reptiles.” The latter term is in scare quotes because biologists consider it a paraphy- letic group (i.e., a group that contains just a subset of the descendants of its common ancestor) that is im- proper for classification purposes due to its exclusion of the birds, which descended from the saurians. Amniotes are characterized by an embryonic membrane that makes terrestrial reproduction feasible. The amphibians, lacking this adaptation, are constrained to place their eggs in an aqueous environment for proper development. These biological details are important because of the nature of some of the evidence that Cabanac et al. bring to bear on the question of consciousness in fish – evidence that I shall maintain seems skewed towards other adaptations that have to do with terrestrial life.