CTRI Trial Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Awareness Among Diabetic Patients
IOSR Journal of Dental and Medical Sciences (IOSR-JDMS) e-ISSN: 2279-0853, p-ISSN: 2279-0861.Volume 15, Issue 10 Ver. VII (October. 2016), PP 39-41 www.iosrjournals.org A Prospective Study -Awareness among Diabetic Patients Dr.S.M.Thirunaukkarasu.M.D1, Dr.S.Sopna Jothi,M.D2, DR.P.Purushothaman,M.D3. Dr.S.Ganesh Babu.M.S4, Dr.T.Ravikumar.M.D5, Dr.P.Saravananm.D6, Dr.Malini,M.D7, Dr.Indhu M.D8. 1Associate Professor Of Medicine Govt Theni Medical College Theni , 2Asst Professor Of Medicine Govt Theni Medical College Theni , 3Professor And. HOD of Medicine Govt Theni Medical College Theni, 4 Professor Of Surgery Govt Theni Medical College Theni , 5Professor and Hod of Medicine, Govt Medical College And ESI HospitalCoimbatore, 6Asst.Professor-of Medicine Madurai Medical College Madurai, 7Asst.Professer Govt Medical College and ESI hospital coimbatore. Abstract: Morbidity and mortality due diabetes in all over world is alarming .We submit a study done over 3 months in the tertiary care hospital in southern part of Tamil nadu Aim Of The Study: The aim of the study is to check the knowledge of the patients prove that educating will improve the outcome. Material And Methods: This is a prospective observational study in a tertiary medical care hospital , in southern part of Tamil Nadu , south India, during september 2016with in 50 patients all are presented with diabetes, on regular treatment (type1 and type 2 on insulin) Results: many patients aware of their disease and management. Majority of them know when to adjust the dose of insulin and our aim is to provide100 percent knowledge Conclusion:. -
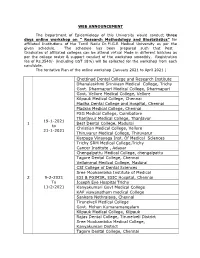
Research Methodology and Biostatistics” for Affiliated Institutions of the Tamil Nadu Dr.M.G.R Medical University As Per the Given Schedule
WEB ANNOUNCEMENT The Department of Epidemiology of this University would conduct three days online workshop on “ Research Methodology and Biostatistics” for affiliated Institutions of the Tamil Nadu Dr.M.G.R Medical University as per the given schedule. The schedule has been prepared such that Post Graduates of affiliated colleges can be attend virtual Mode in different batches as per the college roster & support conduct of the workshop smoothly. Registration fee of Rs.3540/- (including GST 18%) will be collected for the workshop from each candidate. The tentative Plan of the online workshop (January 2021 to April 2021 ) Chettinad Dental College and Research Institute Dhanalaskhmi Srinivasn Medical College, Trichy Govt. Dharmapuri Medical College, Dharmapuri Govt. Vellore Medical College, Vellore Kilpauk Medical College, Chennai Madha Dental College and Hospital, Chennai Madras Medical College, Chennai PSG Medical College, Coimbatore Thanjavur Medical College, Thanjavur 19-1-2021 1 Best Dental College, Madurai to Christian Medical College, Vellore 21-1-2021 Thiruvarur Medical College, Thiruvarur Karpaga Vinayaga Inst. Of Medical Sciences Trichy SRM Medical College,Trichy Cancer Institute , Adayar Chengalpattu Medical College, chengalpattu Tagore Dental College, Chennai Vellammal Medical College, Madurai CSI College of Dental Sciences Sree Mookambika Institute of Medical 29-2-2021 ESI & PGIMSR, ESIC Hospital, Chennai To Joseph Eye Hospital Trichy 11-2-2021 Kanyakumari Govt Medical College KAP viswanatham medical College Sankara Nethralaya, Chennai Tirunelveli Medical College Govt. Mohan Kumaramangalam Kilpauk Medical College, Kilpauk Rajas Dental College, Tirunelveli District Sree Mookambika Medical College, Kanyakumari District Tagore Dental College, Chennai Trichy SRM Medical College,Trichy Vivekanandha Dental College for Women, Namakkal Christian medical College, Vellore Govt. -

Local Bodies of Tamil Nadu Full Report
PREFACE This Report has been prepared for submission to the Governor under Article 151 of the Constitution. 2. This Report sets out the results of audit under the Comptroller and Auditor General of India’s (Duties, Powers and Conditions of Service) Act, 1971, in respect of financial assistance given to urban local bodies. 3. Matters arising from the Finance and Appropriation Accounts for the year 2004-05, together with other points arising out of audit of transactions of the Government of Tamil Nadu are included in a separate volume of the Report (Civil) of 2004-05. 4. The Report containing the observations arising out of audit of Statutory Corporations, Boards and Government Companies and the Report containing such observations on Revenue Receipts are presented separately. 5. The cases mentioned in this Report are among those which came to notice in the course of test check of accounts of local bodies during the year 2004-05, as well as those which had come to notice in earlier years, but could not be dealt with in previous Reports on Government of Tamil Nadu. Matters relating to the period subsequent to March 2005 have also been included, wherever considered necessary. OVERVIEW This Report, dealing with the results of audit of accounts of urban local bodies contains three Performance Reviews and nine Audit Paragraphs. A synopsis of important audit findings is presented in this overview. I Accounts and Finances of Urban Local Bodies There were six municipal corporations and 151 municipalities in Tamil Nadu as on 31 March 2005. The urban population of the State as per 2001 census was 2.75 crore comprising 44 per cent of total State population. -

2018 – 2019 Tamil Nadu Pollution Control Board
Annual Reports & Accounts 2018 – 2019 Tamil Nadu Pollution Control Board 76, Mount Salai, Guindy, Chennai – 600 032 INDEX Chapter Contents Page No. No. 1 Introduction 1 2 Organisational Setup 6 3 Meetings of the Board 11 4 Activities of the Board 19 5 TNPCB Laboratories 57 Air, Water, Noise Quality Monitoring 6 62 Programmes 7 Environmental Standards 71 8 Legal Actions 73 9 Environmental Training Institute 80 Environmental Awareness and Public 10 84 Participation Visits to the Board by Experts, Important 11 88 Delegates and Person Other Important Matters Dealt with by the 12 89 Board 13 Annexures 107 14 Accounts 134 15 Photos 166 CHAPTER – 1 INTRODUCTION 1.1 FORMATION OF TNPCB Government of Tamil Nadu implemented Water (Prevention and Control of Pollution) Act, 1974 (Central Act 6) in Tamil Nadu on 31.08.1981. Based on the Act, the Government in G.O. No. 340 Health and Family Welfare Department dated 19.02.1982 constituted the Tamil Nadu Prevention and Control of Water Pollution Board on 27.02.1982. The Government has declared the entire area within the State of Tamil Nadu as Air Pollution Control areas vide G.O.Ms. No.4, Environment Control Department dated 28.09.1983 under Section 19 (1) of the Air (Prevention and Control of Pollution) Act, 1981. Thereafter in the year 1983, the Tamil Nadu Prevention and Control of Water Pollution Board was renamed as “Tamil Nadu Pollution Control Board (TNPCB)”. 1.2 CONSTITUTION OF THE BOARD According to the provisions of the Water (Prevention and Control of Pollution) Act, 1974, the State Board consists -

Directorate of Medical Education
1 Directorate of Medical Education Hand Book on Right to Information Act - 2005 2 CHAPT ER DETAILS PAGE NO NO. 1. INTRODUCTION 3 2 PARTICULARS OF ORGANISATION, FUNCTIONS AND DUTIES 5 3 POWERS AND DUTIES OF OFFICERS AND EMPLOYEES 80 4 RULES, REGULATIONS,INSTRUCTIONS, MANUAL AND RECORDS 91 FOR DISCHARGING FUNCTIONS 5 PARTICULARS OF ANY ARRANGEMENT THAT EXISTS FOR 93 CONSULTATION WITH OR REPRESENTATION BY THE MEMBERS OF THE PUBLIC IN RELATION TO THE FORMULATION OF ITS POLICY OR IMPLEMENTATION THEREOF 6 A STATEMENT OF THE CATEGORIES OF DOCUMENTS THAT ARE 94 HELD BY IT OR UNDER ITS CONTROL 7 A STATEMENT OF BOARDS, COUNCIL, COMMITTEES AND OTHER 95 BODIES CONSTITUTED AS ITS PART 8 THE NAMES, DESIGNATION AND OTHER PARTICULARS OF THE 96 PUBLIC INFORMATION OFFICER 9 PROCEDURE FOLLOWED IN DECISION MAKING PROCESS 107 10 DIRECTORY OF OFFICERS AND EMPLOYEES 108 11 THE MONTHLY REMUNERATION RECEIVED BY EACH OF ITS 110 OFFICERS AND EMPLOYEES INCLUDING THE SYSTEM OF COMPENSATION AS PROVIDED IN REGULATIONS. 12 THE BUDGET ALLOCATED TO EACH AGENCY/OFFICERS 111 (PARTICULARS OF ALL PLANS, PROPOSED EXPENDITURES AND REPORTS ON DISBURSEMENT MADE 116 13 THE MANNER OF EXECUTION OR SUBSIDY PROGRAMME 14 PARTICULARS OF RECEIPIENTS OF CONCESSIONS, PERMITS OR 117 AUTHORISATION GRANTED BY IT 15 NORMS SET BY IT FOR THE DISCHARGE OF ITS FUNCTIONS 118 16 INFORMATION AVAILABLE IN AN ELECTRONIC FORM 119 17 PARTICULARS OF THE FACILITIES AVAILABLE TO CITIZENS FOR 120 OBTAINING THE INFORMATION 18 OTHER USEFUL INFORMATION 121 3 CHAPTER-1 - INTRODUCTION 1.1This handbook is brought out by the Directorate of Medical Education (Government of Tamil Nadu) , Chennai as required by the Right to Information Act, 2005. -

Executive Summary for Thiru. A. Kumar Rough Stone and Gravel Quarry
Executive Summary Executive Summary For Thiru. A. Kumar Rough stone and Gravel Quarry “B1” Category S.F.Nos 272/3, 272/4, 273/1, 273/2, 273/3, 277/1 & 1240 Extent 7.41.5 Ha Village Kullapuram Taluk Periyakulam District Theni Purpose Prior Environmental Clearance Total Production for 5 Year Mining Plan Rough Stone = 21, 05,265 m3, Period Weathered Formation = 74,514 m3 Gravel = 2, 98,056 m3 Project Cost Rs. 1.40 Crores /- PROPONENT ADDRESS Thiru. A. Kumar, S/o. Ananthakrishnan, No.23, New Sriram Nagar, Allinagaram, Theni - 625 531 PREPARED BY M/s. Geo Exploration and Mining Solutions, Accredited for Sector 1, 28 & 38 Category ‘A’ Quality Council of India – National Accreditation Board for Education & Training, New Delhi Certificate No : NABET/EIA/1821/RA 0123 www.gemssalem.com 1 Executive Summary 1. INTRODUCTION – Thiru. A.Kumar S/o. Ananthakrishnan residing at No.23, New Sriram Nagar, Allinagaram, Theni-625 531 applied for Rough Stone and Gravel quarry lease over an extent of 7.41.5 Ha in S.F.Nos. 272/3, 272/4, 273/1, 273/2, 273/3, 277/1 & 1240 in Kullapuram village, Periyakulam Taluk, Theni District and Tamil Nadu State. The extent of the individual lease is more than 5 Ha ie 7.41.5 ha, This EIA report is prepared to evaluate the environmental impacts of the project in line with the requirements of EIA notification SO 1533(E) dated 14.9.2006 and amendments made thereof. The proposed production of Rough stone is 21,05,265 m3 of Rough Stone, 74,514 m3 of Weathered formation and 2,98,056 m3 of Gravel for five year mining plan period. -

Tamil Nadu Government Gazette Published by Authority
© [Regd. No. TN/CCN/467/2012-14. GOVERNMENT OF TAMIL NADU [R. Dis. No. 197/2009. 2019 [Price : Rs. 1.60 Paise. TAMIL NADU GOVERNMENT GAZETTE PUBLISHED BY AUTHORITY No. 30] CHENNAI, WEDNESDAY, JULY 24, 2019 Aadi 8, Vikari, Thiruvalluvar Aandu – 2050 Part VI—Section 1 Notifications of interest to the General Public issued by Heads of Departments, Etc. NOTIFICations BY HEADS OF Departments, ETC. CONTENTS Pages. GENERAL NOTIFICATIONS Final Closing and Cancellation of Registration of E.H. 213, Kurinchimalar Weavers Co-operative Society in Erode District. .. .. .. .. .. 220 Confirmation of Variation to the Approved Theni-Allinagaram Detailed Development Plan No. V of Theni Allinagaram Local Planning Area .. .. .. .. 220 Variation to the Approved Master Plan for the Mamallapuram Local Planning Area .. 220-221 Thisaiyanvilai Local Planning Authority Notice .. .. .. .. .. 221 Variations to the Approved Master Plan for Kurichi New Town Development Authority 222 [ 219 ] DTP—VI-1-30—1 220 TAMIL NADU GOVERNMENT GAZETTE [Part vi—Sec.1 NOTIFICATIONS BY HEADS OF DEPARTMENTS, ETC. GENERAL NOTIFICationS Final Closing and Cancellation of Registration of E.H. 213, Kurinchimalar Weavers Co-operative Society in Erode District. (C.L. F02/2003/M) No.VI(1)/360/2019. The affairs of the E.H.213 Kurinchimalar Weavers Co-operative Society in Erode District are ordered to be finally closed and its registration cancelled under Section 140(1) of the Tamil Nadu Co-operative Societies Act, 30 of 1983 as per the orders contained in the proceedings of C.L.F02/2003/M, Date: 05-07-2019 of The Assistant Director of Handlooms and Textiles, Erode. Erode, R. -

Para Medical Courses - 2019 - 2020 Session List of Candidates Allotted on 10.09.2019
PARA MEDICAL COURSES - 2019 - 2020 SESSION LIST OF CANDIDATES ALLOTTED ON 10.09.2019 COLLEGE : Madras Medical College, Chennai SNO RANK ARNO NAME TOTAL COMM. COURSE JOIN DATE MARKS 1 1 16111 LUBNA SHERIN 196.50 BCM B.A.S.L.P 2 2 15841 ABDUL RAHMAN S 195.00 BCM B.Pharm 3 4 792 HAMSSIKA S 194.50 OC B.A.S.L.P 4 14 7915 JAYASHREE E 190.75 BC B.Pharm 5 15 139 SURYA PRIYA B 190.50 OC B.Pharm 6 16 644 BOOMIKA T 190.00 BC B.Pharm 7 19A 13725 ANUSHA S 189.50 BC B.Sc.(Nursing) 8 21 9499 RAGUPATHI R 189.50 MBC/DNC B.Pharm 9 23 16586 MOHAN K 189.00 BC B.Sc. Cardio-Pulmonary 10 25 13420 A.ROHINI 189.00 BC B.Sc.(Nursing) 11 26 5613 MUKILAN K 189.00 BC B.A.S.L.P 12 32 14740 SINDHU J A 188.00 MBC/DNC B.Sc. Cardio-Pulmonary 13 34 1394 JEYA SHREE N 187.50 BC B.Sc.(Nursing) 14 36 21067 RANJITHAM M 187.50 MBC/DNC B.Pharm 15 44 2249 VISAKA E M 186.50 MBC/DNC B.Sc. Cardio-Pulmonary 16 46 6664 VENGATESH K G 186.50 MBC/DNC B.Pharm 17 49 21373 ABITHA T 186.25 BC B.Sc.(Nursing) 18 51 1533 AYSHA YASMEEN S 186.00 BCM B.Pharm 19 52 2731 HARI KRISHNAN P 186.00 MBC/DNC B.Pharm 20 56 1416 THAMIL SELVAN R 186.00 BC B.Pharm 21 58 6663 SNEKA S 185.75 BC B.Sc. -

Pg Degree/Diploma Courses 2017 - 2018 Session - on 24.05.2017 List of Candidates Relieved From
PG DEGREE/DIPLOMA COURSES 2017 - 2018 SESSION - ON 24.05.2017 LIST OF CANDIDATES RELIEVED FROM NAME OF THE INSTITUTION : CHENGALPATTU MEDICAL COLLEGE, CHENGALPATTU COMMUNI SERVICE TOTAL ALLOTTED TO PREVIOUS RELIEVED SNO RANK AR.NO NAME TY STATUS MARKS SPECIALITY DATE CURRENT SPECIALITY CURRENT COLLEGE 1 74 52537 Dr.CHILAMBARASAN V BC SERV 1301.945320 M.D. Paediatrics THANJAVUR MEDICAL M.D. Paediatrics COLLEGE, THANJAVUR 2 82 53545 Dr.VEERAMANI C BC SERV 1295.744320 M.D. General GOVT. MOHAN M.D. Paediatrics Medicine KUMARAMANGALAM MEDICAL 3 119 53262 Dr.B PRIYANKA OC SERV 1273.930680 M.S. Obstetrics MADRAS MEDICAL COLLEGE, M.S. Obstetrics and and Gynaecology CHENNAI Gynaecology 4 158 51494 Dr.ISHWARYA R OC SERV 1245.083280 M.D. General KILPAUK MEDICAL COLLEGE M.D. General Medicine , CHENNAI Medicine 5 253 53623 Dr.SIDDHARTHAN R BC SERV 1208.677920 M.D. General CHENGALPATTU MEDICAL M.S. General Medicine COLLEGE, CHENGALPATTU Surgery 6 264 53620 Dr.L LALITHKUMAR BC SERV 1204.666450 M.S. General STANLEY MEDICAL COLLEGE M.S. General Surgery , CHENNAI Surgery 7 281 54539 Dr.KALAIYARASI BC SERV 1198.640640 M.S. Obstetrics GOVT. MOHAN M.S. Obstetrics and and Gynaecology KUMARAMANGALAM MEDICAL Gynaecology 8 369 52105 Dr.NIRMAL THEJRAJ OC SERV 1167.247510 M.D. MADRAS MEDICAL COLLEGE, M.D. Anaesthesiology CHENNAI Anaesthesiology 9 388 53137 Dr.SENTHIL RAJA V MBC/DNC SERV 1163.340490 M.S. General THANJAVUR MEDICAL M.S. General Surgery COLLEGE, THANJAVUR Surgery 10 429 51471 Dr.THENDRALARASI R BC SERV 1153.893780 M.D. STANLEY MEDICAL COLLEGE M.D. -

State District Branch Address Centre Ifsc Contact1 Contact2 Contact3 Micr Code
STATE DISTRICT BRANCH ADDRESS CENTRE IFSC CONTACT1 CONTACT2 CONTACT3 MICR_CODE ANDAMAN NO 26. MG ROAD AND ABERDEEN BAZAR , NICOBAR PORT BLAIR -744101 704412829 704412829 ISLAND ANDAMAN PORT BLAIR ,A & N ISLANDS PORT BLAIR IBKL0001498 8 7044128298 8 744259002 UPPER GROUND FLOOR, #6-5-83/1, ANIL ANIL NEW BUS STAND KUMAR KUMAR ANDHRA ROAD, BHUKTAPUR, 897889900 ANIL KUMAR 897889900 PRADESH ADILABAD ADILABAD ADILABAD 504001 ADILABAD IBKL0001090 1 8978899001 1 1ST FLOOR, 14- 309,SREERAM ENCLAVE,RAILWAY FEDDER ROADANANTAPURA ANDHRA NANTAPURANDHRA ANANTAPU 08554- PRADESH ANANTAPUR ANANTAPUR PRADESH R IBKL0000208 270244 D.NO.16-376,MARKET STREET,OPPOSITE CHURCH,DHARMAVA RAM- 091 ANDHRA 515671,ANANTAPUR DHARMAVA 949497979 PRADESH ANANTAPUR DHARMAVARAM DISTRICT RAM IBKL0001795 7 515259202 SRINIVASA SRINIVASA IDBI BANK LTD, 10- RAO RAO 43, BESIDE SURESH MYLAPALL SRINIVASA MYLAPALL MEDICALS, RAILWAY I - RAO I - ANDHRA STATION ROAD, +91967670 MYLAPALLI - +91967670 PRADESH ANANTAPUR GUNTAKAL GUNTAKAL - 515801 GUNTAKAL IBKL0001091 6655 +919676706655 6655 18-1-138, M.F.ROAD, AJACENT TO ING VYSYA BANK, HINDUPUR , ANANTAPUR DIST - 994973715 ANDHRA PIN:515 201 9/98497191 PRADESH ANANTAPUR HINDUPUR ANDHRA PRADESH HINDUPUR IBKL0001162 17 515259102 AGRICULTURE MARKET COMMITTEE, ANANTAPUR ROAD, TADIPATRI, 085582264 ANANTAPUR DIST 40 ANDHRA PIN : 515411 /903226789 PRADESH ANANTAPUR TADIPATRI ANDHRA PRADESH TADPATRI IBKL0001163 2 515259402 BUKARAYASUNDARA M MANDAL,NEAR HP GAS FILLING 91 ANDHRA STATION,ANANTHAP ANANTAPU 929710487 PRADESH ANANTAPUR VADIYAMPETA UR -

List of Food Safety Officers
LIST OF FOOD SAFETY OFFICER State S.No Name of Food Safety Area of Operation Address Contact No. Email address Officer /District ANDAMAN & 1. Smti. Sangeeta Naseem South Andaman District Food Safety Office, 09434274484 [email protected] NICOBAR District Directorate of Health Service, G. m ISLANDS B. Pant Road, Port Blair-744101 2. Smti. K. Sahaya Baby South Andaman -do- 09474213356 [email protected] District 3. Shri. A. Khalid South Andaman -do- 09474238383 [email protected] District 4. Shri. R. V. Murugaraj South Andaman -do- 09434266560 [email protected] District m 5. Shri. Tahseen Ali South Andaman -do- 09474288888 [email protected] District 6. Shri. Abdul Shahid South Andaman -do- 09434288608 [email protected] District 7. Smti. Kusum Rai South Andaman -do- 09434271940 [email protected] District 8. Smti. S. Nisha South Andaman -do- 09434269494 [email protected] District 9. Shri. S. S. Santhosh South Andaman -do- 09474272373 [email protected] District 10. Smti. N. Rekha South Andaman -do- 09434267055 [email protected] District 11. Shri. NagoorMeeran North & Middle District Food Safety Unit, 09434260017 [email protected] Andaman District Lucknow, Mayabunder-744204 12. Shri. Abdul Aziz North & Middle -do- 09434299786 [email protected] Andaman District 13. Shri. K. Kumar North & Middle -do- 09434296087 kkumarbudha68@gmail. Andaman District com 14. Smti. Sareena Nadeem Nicobar District District Food Safety Unit, Office 09434288913 [email protected] of the Deputy Commissioner , m Car Nicobar ANDHRA 1. G.Prabhakara Rao, Division-I, O/o The Gazetted Food 7659045567 [email protected] PRDESH Food Safety Officer Srikakulam District Inspector, Kalinga Road, 2. K.Kurmanayakulu, Division-II, Srikakulam District, 7659045567 [email protected] LIST OF FOOD SAFETY OFFICER State S.No Name of Food Safety Area of Operation Address Contact No. -

District Statistical Handbook of 2008-2009. Theni District.Pdf
DISTRICT STATISTICAL HAND BOOK OF 2008-2009 THENI DISTRICT 1. Area and Population 17. Industries 33. Registration 2. Rainfall and Climate 18. Factories 34. Repairs and Services 3. Agriculture 19. Local Bodies 35. Restaurants and Hotels 4. Irrigation 20. Labour and Employment 36. Cultural Services 5. Animal Husbandry 21. Legal Services 37. Social Welfare 6. Banking and Insurance 22. Libraries 38. Sanitary Services 7. Co-operation 23. Mining and Quarring 39. Scientific and Research 8. Civil Supplies 24. Manufacturing Sector 40. Storage Facility 9. Communication 25. Medical Services 41. Textiles 10. Electricity 26. Motor Vehicles 42. Trade and Commerce 11. Education 27. Energy 43. Transport 12. Fisheries 28. Police and Prisons 44. Tourism 13. Handloom 29. Public Health 45. Vital Statistics 14. Handicrafts 30. Publication and Printing 46. Voluntary Services 15. Hospitals 31. Price-Indices 47. Water Supply 16. Housing 32. Quality Control DISTRICT STATISTICAL HAND BOOK 2008-2009 THENI DISTRICT 2001-POPULATION TALUK NAME MALE FEMALE TOTAL Theni 88268 85877 174145 Andipatti 95334 91236 186570 Periyakulam 95629 92925 188554 Bodinaickanur 84484 83234 167718 Uthamapalayam 189271 187692 376963 Total 552986 540964 1093950 MUNICIPALITY Theni 43274 42224 85498 Periyakulam 21104 20908 42012 Bodinaickanur 36774 36636 73410 Chinnamanur 19285 19075 38360 Cumbum 29515 29376 58891 Gudalore 17810 17721 35531 TOWN PANCHAYATS Boothipuram 4903 4723 9626 Meenatchipuram 3634 3593 7227 Melachokanathapuram 5862 5807 11669 Devathanapatti 6942 7009 13951 Ganguvarpatti