TB Monograph
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Msinga Experience Lessons Learnt from South Africa 2005–2009 Contents
TUBERCULOSIS MDR/XDR The Msinga Experience Lessons learnt from South Africa 2005–2009 Contents Summary 2 Introduction 4 The MDR/XDR TB epidemic 7 Addressing MDR/XDR TB 10 Turning the tide 15 MDR/XDR TB cases decrease 17 The way forward 20 Endnotes 21 Published in partnership with Umzinyathi District Management January 2009 TUBERCULOSIS MDR/XDR The Msinga Experience Lessons learnt from South Africa 2005–2009 © Pg-images/Dreamstime.com 1 Factors behind the decline in drug resistant TB in Msinga: Summary • The commitment of the Umzinyathi health district management team ensured that TB control was placed at the top of Drug resistant tuberculosis has emerged as a serious public the district’s agenda and that adequate resources health issue around the world. Recent global estimates put were allocated to tackle the number of reported cases for 2006 at close to half a the disease. million. This represents 4.8 percent of all notified TB cases • The management of TB worldwide. An estimated 1.5 million people died from TB in patients was aggressively 2006. addressed by providing In 2006, an outbreak of a deadly and almost incurable refresher training for form of TB was reported in Msinga, Umzinyathi district, a nurses and introducing remote and rural part of KwaZulu-Natal province in South appointment diaries to Africa. The extensively drug-resistant TB or ‘XDR TB’ as it track patients. became known largely occurred among HIV-infected • An increase in the nurse- people, in particular those with terminal AIDS. to-patient ratio also played Patients who were co-infected with XDR TB and HIV an important role in improv- stood little chance of survival. -
Protocol for the Care of Patients with Tuberculosis
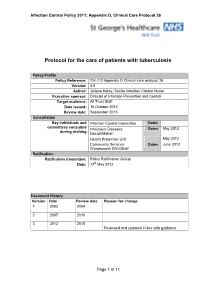
Infection Control Policy 2011; Appendix D, Clinical Care Protocol 26 Protocol for the care of patients with tuberculosis Policy Profile Policy Reference: Clin.2.0 Appendix D Clinical care protocol 26 Version: 3.0 Author: Juliana Kotey, Senior Infection Control Nurse Executive sponsor: Director of Infection Prevention and Control Target audience: All Trust Staff Date issued: 16 October 2012 Review date: September 2015 Consultation Key individuals and Infection Control Committee Dates committees consulted Infectious Diseases Dates May 2012 during drafting Doctor/Matron Health Protection Unit May 2012 Community Services Dates June 2012 Wandsworth DDN/Staff Ratification Ratification Committee: Policy Ratification Group Date: 17 th May 2012 Document History Vers ion Date Review date Reason for change 1 2002 2004 2 2007 2010 3 2012 2015 Reviewed and updated in line with guidance. Page 1 of 11 Infection Control Policy 2011; Appendix D, Clinical Care Protocol 26 Contents Paragraph Page Executive Summary 3 Scope 3 1 Introduction 4 2 Mode of Transmission 4 3 Risk of Transmission 4 3 Non - Respiratory Tract Tuberculosis (Closed TB) 5 4 Respiratory Tract Tuberculosis (Open TB) - Sensitive Strains 5 Respiratory Tract Tuberculosis (Open TB) – Multi Drug 5 Resistant Strains (MDR TB) and Extensively Drug Resistant 6 (XDR TB) 6 Induced Sputum Specimens – sensitive and MDR TB 7 7 Contact Tracing 8 8 Incident Management 8 9 Associated Documents 9 10 References 10 Appendix A Algorithm 1 TB Care Pathway 11 Page 2 of 11 Infection Control Policy 2011; Appendix D, Clinical Care Protocol 26 Executive Summary This document is an evidence-based protocol for the implementation of sound tuberculosis (TB) infection control by all healthcare workers. -

Tuberculosis in Infants Less Than 3 Months Ofage
Archives of Disease in Childhood 1993; 69: 371-374 371 Tuberculosis in infants less than 3 months of age Arch Dis Child: first published as 10.1136/adc.69.3.371 on 1 September 1993. Downloaded from H S Schaaf, R P Gie, N Beyers, N Smuts, P R Donald Abstract identified from a register of cases proved by The clinical and radiological features in 38 culture. infants less than 3 months of age with A history of contact with adult pulmonary tuberculosis proved by culture are tuberculosis, the presenting symptoms and described and may aid early diagnosis of their duration, and clinical features such as this often fatal condition. Respiratory lymphadenopathy, respiratory signs, and the symptoms, cough in 33 (87%) and tachyp- presence of hepatosplenomegaly were noted. noea in 31 (82%), were the commonest Tuberculin testing was either by Mantoux test presenting symptoms. Twenty five infants 5 units purified protein derivative or Tine test (66%) had hepatomegaly and 20 (53%) (Lederle) with an induration of > 15 mm or a splenomegaly. Mantoux testing gave an confluent reaction respectively being regarded induration of >15 mm in three of 17 (18%) as significant. infants. In a further five a Tine test gave The chest radiographs of 27 (71%) of the 38 confluent response. Chest radiography in infants were assessed systematically by a panel 27 infants showed miliary tuberculosis in consisting of all the authors. Particular seven (26%) and hilar or paratracheal attention was paid to the presence of miliary adenopathy in 14 (52%) and 10 (37%) tuberculosis, the presence of hilar or para- respectively. -

BTS Guidelines Control and Prevention of Tuberculosis
Thorax 2000;55:887–901 887 BTS Guidelines Thorax: first published as 10.1136/thorax.55.11.887 on 1 November 2000. Downloaded from Control and prevention of tuberculosis in the United Kingdom: Code of Practice 2000 *Subcommittee comprising Peter Joint Tuberculosis Committee of the British Thoracic Society* Ormerod, Royal Infirmary Blackburn (Chairman, Joint Tuberculosis Committee); Craig Keywords: tuberculosis; BTS guidelines; code of Skinner, Heartlands Abstract practice Hospital, Birmingham; Background—The guidelines on control John Moore-Gillon, St and prevention of tuberculosis in the Bartholomew’s and The United Kingdom have been reviewed and Introduction/evidence criteria Royal London Hospitals, updated. London; Peter Davies, Since publication of the previous control and Aintree University Methods—A subcommittee was appointed prevention guidelines in 19941 new data have Hospital, Liverpool; by the Joint Tuberculosis Committee become available in a number of areas, Mary Connolly, Chest (JTC) of the British Thoracic Society to particularly in infection control, bovine tuber- Clinic, Birmingham revise the guidelines published in 1994 by culosis, and the risks of transmission of tuber- (representing Royal the JTC, including representatives of the culosis during air travel which have brought College of Nursing Royal College of Nursing, Public Health Tuberculosis Special requests for advice. The epidemiology of Interest Group); Virginia Medicine Environmental Group, and tuberculosis in Britain has continued to change Gleissberg, Chest Clinic, Medical Society for Study of Venereal in recent years. Newham, London Diseases. In preparing the revised guide- The numbers of notified cases in England (representing Royal lines the authors took account of new pub- and Wales, which had declined to 5085 in College of Nursing lished evidence and graded the strength of Tuberculosis Special 1987, rose to 5798 in 1992 and 6087 in 1998. -

Diagnosis of Tb
DIAGNOSIS OF TB DR. KONG PO MARN KONG CLINIC FOR CHEST & INTERNAL MEDICINE PATHOGENESIS OF TB INFECTION AND DISEASE (I) PATHOGENESIS OF TB INFECTION AND DISEASE (II) MODES OF DIAGNOSIS • Microbiologic • Smear • Culture • Others ( MODS etc) • Molecular • Nucleic Amplification Assays (NAA) • IFN-γRelease Assays (IGRA) • Line-probe PCR for drug sensitivity testing • Urinary Assays CURRENT PROBLEMS • Active disease • Smears are insensitive • NTM make up 5% of positive smears locally • Cultures take too long ( 12 to 14 days to be positive) • Latent TB Infection (LTBI) • Mantoux test since 1981 • BCG, NTM and boosting can confound issue • Low sensitivity and specificity • Repeat visits NEW MODALITIES IN THE DIAGNOSIS OF ACTIVE TB • NAA • Brochoalveolar lavage with Elispot • Urinary assays ? NUCLEIC AMPLIFICATION TESTS/PCR • Rationale • Isolation issues • Issues with current TB testing • MDR-TB • Standardized and come in kit forms NAA • Amplicor: DNA PCR • Amplified / Enhanced MTD / MTA: rRNA detection • Both FDA approved • In 1999, E-MTD approved for use in both smear positive and negative specimens NAA • Sensitivity is markedly improved cf. smears • MTD: Sensitivity usually range from 83 to 98% in smear + cases. 70 to 81% in smear negative cases. Amplicor slighly less • Specificity is 98 to 99% • Negative predictive value is more important NAA • E-MTD / MTA • Improved sensitivity in smear negative cases • 1999 study. 489 inmates in Texas prison • Overall sensitivity of 95.2% and specificty of 99.1%. • 100% in smear positive cases • 90.2% and 99.1% in smear negative cases • Ontario study (1999) using clinical diagnosis as reference gave nearly 100% specificity and sensitivity for both smear + and sm- cases (Case selection !!) Figure 2. -

The Putrefied Body Niques Require Care but Little Skill to Perform
1300 BRITISH MEDICAL JOURNAL 19 MAy 1979 hospital staffing. All too often, however, reasonable proposals in the tine test), the repeatability of the results, and their for reform are not put into practice, and the same may happen comparability with those of the Mantoux test. here. Consultants will feel threatened: some will lose junior Ideally we should have a single screening test of tuberculin Br Med J: first published as 10.1136/bmj.1.6174.1300 on 19 May 1979. Downloaded from staffand long term the quality ofconsultant work will change- sensitivity that is simple, reliable, easily read, repeatable, safe, and inevitably there will be a sharpening of the contrasts and cheap. None has proved supreme in practice. The many between teaching and non-teaching hospitals and between comparisons of the Mantoux with the multiple-puncture popular and shortage specialties. Junior staff will not like the techniques have given confficting results. In 1959 a report of restriction on their freedom of choice; and overseas doctors the Research Committee of the British Tuberculosis Associa- will complain that they are being singled out for special tion2 concluded that, for epidemiological use, the Heaf treatment. multiple-puncture test would be preferable to the Mantoux 5 All these groups must surely recognise that changes are TU test because of its greater sensitivity; the Heaf test gave overdue, and if they reject the proposals they have an obliga- results intermediate between 5 TU and 100 TU Mantoux tion to suggest alternatives. If there is too much opposition, tests. In 1964 Emerson3 compared the Heaf and tine tests and however, committees that should be taking unpopular deci- found that more than 15% of tuberculin-positive reactors were sions may not do so. -

TB Policies in 24 Countries a Survey of Diagnostic and Treatment Practices About Médecins Sans Frontières
Out of Step 2015 TB Policies in 24 Countries A survey of diagnostic and treatment practices About Médecins Sans Frontières Médecins Sans Frontières (MSF) is an independent international medical humanitarian organisation that delivers medical care to people affected by armed conflicts, epidemics, natural disasters and exclusion from healthcare. Founded in 1971, MSF has operations in over 60 countries today. MSF has been involved in TB care for 30 years, often working alongside national health authorities to treat patients in a wide variety of settings, including chronic conflict zones, urban slums, prisons, refugee camps and rural areas. MSF’s first programmes to treat multidrug-resistant TB opened in 1999, and the organisation is now one of the largest NGO treatment providers for drug-resistant TB. In 2014, the organisation started 21,500 patients on first-line TB treatment across projects in more than 20 countries, with 1,800 patients on treatment for drug-resistant TB. About the MSF Access Campaign In 1999, on the heels of MSF being awarded the Nobel Peace Prize – and largely in response to the inequalities surrounding access to HIV/AIDS treatment between rich and poor countries – MSF launched the Access Campaign. Its sole purpose has been to push for access to, and the development of, life- saving and life-prolonging medicines, diagnostics and vaccines for patients in MSF programmes and beyond. About Stop TB Partnership The Stop TB Partnership is leading the way to a world without TB, a disease that is curable but still kills three people every minute. Founded in 2001, the Partnership’s mission is to serve every person who is vulnerable to TB and to ensure that high-quality treatment is available to all who need it. -

Health Care Provider Information
Health Care Provider Information NOTE: This page is a resource for Whatcom County clinicians dealing with tuberculosis: recognizing active disease and screening for and treating latent infection. The Whatcom County Health Department provides this as a tool/resource in our collaboration with community clinicians, and we welcome feedback and suggestions for additions and changes that improve its usefulness. (Contact Info) Distinguishing Active TB Disease from Latent Infection TB life cycle/Transmission: Tuberculosis (TB) is a disease caused by bacteria (Mycobacteria tuberculosis, or Mtb) transmitted from people with active pulmonary or laryngeal TB disease as aerosolized particles that are suspended in air. Other people may inhale these infectious particles and become infected with Mtb. In the vast majority of cases, their immune system responds to the infection and walls it off, resulting in a latent tuberculosis infection with no disease (and not contagious). When the infected person is immunosuppressed (e.g., HIV/AIDS) or has an immature immune system (young child), the infection may progress to primary active disease without latency. Immune response: The asymptomatic, noncontagious cases are identified by measuring their immune response to tuberculosis, using a TB skin test (TST) or a blood test (interferon gamma release assay, or IGRA). Progression to disease: About 5% of those with latent infection progress to active tuberculosis disease in the first two years after becoming infected. The risk for progression after that is about 0.1% per year (1% per decade of remaining lifetime). The risk of progression increases with conditions that suppress the immune system. This progression can occur with initial infection (primary disease) or after a latent period (reactivation disease). -

Chapter 5 Diagnosis of Latent Tuberculosis Infection (LTBI)
Chapter 5 Diagnosis of Latent Tuberculosis Infection (LTBI) CONTENTS Introduction ............................................. 5.2 Purpose................................................................ 5.2 Policy ................................................................... 5.2 Tuberculosis Classification System ..... 5.3 High-Risk Groups ................................... 5.4 Diagnosis of Latent Tuberculosis Infection ........................... 5.5 Interferon gamma release assays ........................ 5.5 Selecting a TB screening Test ............................. 5.6 Mantoux tuberculin skin testing ........................... 5.7 Candidates for Mantoux tuberculin skin testing ........................................................... 5.8 Administration of the tuberculin skin test ........... 5.11 Measurement of the tuberculin skin test ........... 5.13 Interpretation of the tuberculin skin test ............. 5.14 Human immunodeficiency virus screening ........ 5.16 Follow-up activities ............................................. 5.16 Chest radiography .............................................. 5.16 Resources and References ................. 5.19 NEVADA TUBERCULOSIS PROGRAM MANUAL Diagnosis of Latent Tuberculosis Infection 5 . 1 R e v i s e d A P R I L 2 0 1 8 Introduction Purpose Use this section to understand and follow national and Nevada State guidelines to do the following: ▪ Classify patients with latent TB infection (LTBI). ▪ Diagnose LTBI. In the 2005 guideline “Controlling Tuberculosis in the United States: -

Tuberculosis
Eye (2006) 20, 1068–1073 & 2006 Nature Publishing Group All rights reserved 0950-222X/06 $30.00 www.nature.com/eye 1 1 1 1 CASE SERIES Tuberculosis: an D Varma , S Anand , AR Reddy , A Das , JP Watson1, DC Currie2, I Sutcliffe2 and under-diagnosed OC Backhouse1,2 aetiological agent in uveitis with an effective treatment Abstract around the eye, or on its surface. Classically, ocular TB has been divided into two types: Purpose To highlight the diversity of clinical primary and secondary. Primary disease implies presentations with tubercular uveitis in a that the eye is the initial port of entry while in nonendemic setting, and discuss the secondary disease the organisms spread to the diagnostic approach and an effective eye haematogenously. Primary disease includes treatment. conjunctival, corneal, and scleral disease, while Method Descriptive case series. tuberculous uveitis is a manifestation of Results A total of 12 cases of varied secondary disease. The most common clinical presentations of tubercular uveitis diagnosed findings of intraocular TB include solitary or over a period of 1 year of which six cases are multiple choroidal nodules, choroiditis, and described in detail. Presentations included retinal vasculitis.2–5 The choroidal nodules choroidal tuberculomas, multifocal suggest direct haematogenous infection while choroiditis, recurrent granulomatous uveitis, the vasculitis and choroiditis are more likely to panuveitis with cystoid macular oedema, and be the result of immune hypersensitivity. serpiginous choroiditis. All cases had a Anterior tuberculous uveitis is typically chronic or recurrent course and responded granulomatous and an accompanying vitritis is very well to antitubercular treatment. not uncommon.2 TB should be considered in the Diagnosis was mainly assisted by positive differential diagnosis of chronic anterior tuberculin testing. -

Tuberculin Positive Children Thorax: First Published As 10.1136/Thx.47.10.768 on 1 October 1992
768 Thorax 1992;47:768-769 Tuberculin positive children Thorax: first published as 10.1136/thx.47.10.768 on 1 October 1992. Downloaded from Children have been routinely tuberculin tested as a screen- which detected 27 of the 45 notifications in the survey. ing procedure during the school BCG vaccination When the first year was excluded those with grade 3 and 4 programme in Britain since 1952. The test is generally Heafreactions had an annual notification rate of 1-61/1000; made with the Heafmultiple puncture apparatus. The fixed the corresponding rate for grade 2 reactors was 0-43. head Heaf gun requires disinfection by immersion in During 1970-83 5380 positive reactors were surveyed.5 alcohol followed by ignition of the spirit before each test to Initial chest radiographs yielded 10 cases, only five more prevent cross infection.' A new disposable head apparatus cases being notified during subsequent follow up.6 The ratio (Bignell 2000) is now available and is the method of choice of new cases detected to initial radiographs taken was 1:555 as it eliminates the risk of crossinfection and is simple and for grade 2 reactors, 1:75 for grade 3, and 1:25 for grade 4. accurate in tus is appve joint We may reasonable conclude from the results of these Committee on Vaccination and Immunisation and an studies that for previously unvaccinated children of white explanatory video has been produced by the Department of ethnic origin who are grade 3 and 4 reactors an initial Health.2 The proportion of schoolchildren aged 1013 examination and chest radiograph is valuable. -

Tuberculin Testing: Its Basis, Methods, and Comparative Results
Tuberculin testing: Its basis, methods, Tuberculin test and comparative results DOUGLAS P. HAGEN, D.O. Kirksville, Mo. Several procedures for administering test has converted to positive. Several methods tuberculin for skin testing of performing tuberculin skin tests will be re- viewed, and the results of the methods will be are reviewed. The Mantoux test is compared. considered the most accurate, in that the test dose can be measured Basis for tuberculin testing accurately, the equipment and During the first infection with the tubercle materials required are available readily, bacillus, the host acquires hypersensitivity to the organism. Hypersensitivity can be induced and the reactions can be read by the whole tubercle bacillus alone or a and recorded quantitatively. Its chief combination of the wax fraction of the or- disadvantage is the skill required ganism containing a lipopolysaccharide and to administer the test. Multiple-puncture tuberculoprotein but not by tuberculoprotein techniques need minimal skill alone. The reaction that occurs when an anti- for administration and false negative gen to tuberculosis is introduced intradermally provides a valuable testing procedure for both reactions are rare. However, the epidemiologic and case finding purposes. tuberculins are not well standardized, Tuberculin testing material is basically of the dose of tuberculin two types, old tuberculin (OT) and purified retained in the skin is unknown, and protein derivative (PPD). Old tuberculin is false positive reactions may a concentrated filtrate of broth in which tu- occur. Because of the variables affecting bercle bacilli have grown for 6 weeks. Cultures of Mycobacterium tuberculosis are heat-ster- the dose, quantitative measures ilized.