Avian Biodiversity in the Guessabo Wetland, Centre
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ornithological Expedition to Southern Bénin, April 2011
Ornithological expedition to southern Bénin, April 2011 Françoise Dowsett-Lemaire & Robert J. Dowsett Dowsett-Lemaire Misc. Report 80 (2011) Dowsett-Lemaire F. & Dowsett R.J. 2011. Ornithological expedition to southern Bénin, April 2011. Dowsett-Lemaire Miscellaneous Report 80: 16pp. Birds of southern Bénin -1- Dowsett-Lemaire Misc. Rep. 80 (2011) Ornithological expedition to southern Bénin, April 2011 by Françoise Dowsett-Lemaire & Robert J. Dowsett Résumé Ceci est notre deuxième visite au sud du Bénin, faisant suite à une première expédition pendant la saison sèche de 2009. Le mois d’avril 2011 a été partagé entre les forêts principales du sud (Lama, Niaouli), les forêts plus sèches ou forêts claires des Monts Kouffé et Tobé à l’ouest, les plaines marécageuses du Zou et du Sô, et la zone côtière (Pahou et Grand-Popo). Un séjour du 12 au 16 avril à la limite sud des Monts Kouffé et dans la zone protégée de Tobé (Bantè) a permis d’étendre l’aire de distribution de plusieurs espèces forestières ou de sa - vane, notamment du rare Aigle couronné Stephanoaetus coronatus (un ex. essayant de capturer un Daman des rochers sur le rocher de Tobé), du Râle perlé Sarothrura pulchra (un chanteur à Tobé, limite nord actuelle), de l’Oedicnème tachard Burhinus capensis (Tobé, limite sud actuelle), Malcoha à bec jaune Ceuthmochares aereus (Tobé & Kouffé, limite nord), Coucal à ventre blanc Centropus leucogaster (Tobé & Kouffé, limite nord), Grand-duc de Verreaux Bubo lacteus (Kouffé), Trogon narina Apaloderma narina (Kouffé), Calao rieur By - canistes -

Ghana Mega Rockfowl & Upper Guinea Specials 3 to 25 January 2016 (23 Days) Trip Report
Knox Ghana Mega Rockfowl & Upper Guinea Specials 3 to 25 January 2016 (23 days) Trip Report Akun Eagle-Owl by David Hoddinott Trip Report compiled by Tour Leader Markus Lilje RBT Knox Ghana Mega Trip Report January 2015 2 Trip Summary Our private Ghana Mega trip proved yet again to be a resounding success! We notched up a fantastic species total in 23 days, where we covered the length and breadth of the country and a great variety of habitats in this superb West African country! Our tour started off with a visit to Shai Hills. This small but fabulous reserve has a nice variety of habitats including mixed woodland, grassland, wetlands and granite outcrops and therefore supports an interesting array of bird species. During our morning exploring the reserve we recorded African Cuckoo-Hawk, Western Marsh Harrier, Red-necked Buzzard, stunning Violet Turaco, numerous immaculate Blue-bellied Roller, Vieillot’s and Double-toothed Barbets, Senegal and African Wattled Lapwings, White-shouldered Black Tit, Red- shouldered Cuckooshrike, Black-bellied Bustard, Senegal Parrot, Senegal Batis and restless Senegal Eremomela. A number of migrants were seen including Willow Warbler, Whinchat and Spotted Flycatcher. Even mammals showed well for us as we had a number of Kob, Bushbuck, Olive Baboon, Callithrix Monkey and unusually good views of Lesser Spot- Blue-bellied Roller by Markus Lilje nosed Monkey! Well pleased with our morning’s birding, we left Shai Hills and made our way to Ho. En route we stopped for lunch near the Volta Dam where we enjoyed most memorable close-up encounters with Mangrove Sunbird and Bronze- tailed Starling. -

Le Developpement De La Peche En Côte D'ivoire
LE DEVELOPPEMENT DE LA PECHE EN CÔTE D’IVOIRE : LE CAS DE LA PECHE CONTINENTALE DANS LA SOUS-PREFECTURE DE GUESSABO KRA koffi siméon Assistant , [email protected] Université Jean Lorougnon Guédé de Daloa / Côte d’Ivoire RÉSUMÉ ABSTRACT Située dans le centre ouest ivoirien, la sous-préfecture de Situated in the west center of the Ivory Coast, the under-pre- Guessabo présente de nombreux atouts pour le développement fecture of Guessabo presents many assets for the development de la pêche. Et pourtant, les politiques en matière de pêche en of the fishing. And yet, the policies concerning fishing in Coast of Côte d’Ivoire ont souvent mis l’accent sur les pêches maritimes Ivory often put the accent on the maritime fishings and lagunaires. et lagunaires. Le but de cette étude est d’évaluer les potentialités The goal of this survey is to value the piscatorial potentialities halieutiques de la sous-préfecture de Guessabo, d’analyser la of the under-prefecture of Guessabo, to analyze the structure structure sociodémographique des acteurs de la pêche et enfin sociodémographique of the actors of the fishing and finally to d’identifier les problèmes auxquels sont confrontés les acteurs de identify the problems to which are confronted the actors of the la filière pêche de cette localité. path fishes this locality. Plusieurs techniques de recueil d’informations ont été Several techniques of compilation of information have been combinées pour y parvenir. Ce sont entre autre la recherche combined to arrive there. These are between other the documen- documentaire, l’observation, l’enquête par le questionnaire et tary research, the observation, the investigation by the question- des guides d’entretien avec les acteurs intervenant dans la filière. -

Bird Checklists of the World Country Or Region: Ghana
Avibase Page 1of 24 Col Location Date Start time Duration Distance Avibase - Bird Checklists of the World 1 Country or region: Ghana 2 Number of species: 773 3 Number of endemics: 0 4 Number of breeding endemics: 0 5 Number of globally threatened species: 26 6 Number of extinct species: 0 7 Number of introduced species: 1 8 Date last reviewed: 2019-11-10 9 10 Recommended citation: Lepage, D. 2021. Checklist of the birds of Ghana. Avibase, the world bird database. Retrieved from .https://avibase.bsc-eoc.org/checklist.jsp?lang=EN®ion=gh [26/09/2021]. Make your observations count! Submit your data to ebird. -

Sierra Leone
SIERRA LEONE 9 - 24 FEBRUARY 2008 TOUR REPORT LEADER: NIK BORROW Our first exploratory tour to Sierra Leone was pretty tough going at times but certainly pulled a few goodies out of the bag! A respectable total of 305 species were recorded of which all but 12 were seen. The notable major highlights had to be the wonderful views of the amazing Yellow-headed Picathartes preening and posing at their nest site before going to roost, the restricted range Turati’s Boubou and no less than four stunning Gola Malimbes for everyone! Singing Brown Nightjars were discovered, sublime Egyptian Plovers enjoyed, colourful Buff-throated Sunbirds enthralled and secretive Capuchin Babblers were tracked down. Mammals were sparse but we had great looks at the beautiful Diana Monkey and Olive Colobus and we even almost saw a Pygmy Hippo that crashed away from us through the undergrowth! Other specialties included Red-chested Goshawk, Latham’s Forest Francolin, Black-shouldered and Standard-winged Nightjars, Blue-headed Bee-eater, Brown- cheeked and Yellow-casqued Hornbills, Hairy-breasted Barbet, Spotted Honeyguide, Little Green, Melancholy and Fire-bellied Woodpeckers, Fanti Saw-wing, Preuss’s Cliff Swallow, Pied-winged Swallow, Green-tailed and Grey-headed Bristlebills, Western Bearded Greenbul, Yellow-bearded Greenbul, Western Forest Robin, White-tailed Alethe, Finsch’s Flycatcher Thrush, Forest Scrub Robin, Sharpe’s Apalis, Kemp’s Longbill, Olivaceous and Ussher’s Flycatchers, Red-cheeked Wattle-eye, Rufous-winged and Puvel’s Illadopsis, Red-billed Helmet-shrike, Copper-tailed Glossy and Emerald Starlings, Maxwell’s Black Weaver, Red-vented Malimbe, Yellow-winged Pytilia and Dybowski’s Twinspot. -
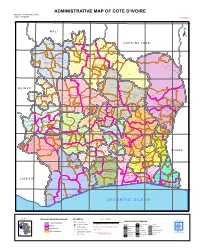
ADMINISTRATIVE MAP of COTE D'ivoire Map Nº: 01-000-June-2005 COTE D'ivoire 2Nd Edition
ADMINISTRATIVE MAP OF COTE D'IVOIRE Map Nº: 01-000-June-2005 COTE D'IVOIRE 2nd Edition 8°0'0"W 7°0'0"W 6°0'0"W 5°0'0"W 4°0'0"W 3°0'0"W 11°0'0"N 11°0'0"N M A L I Papara Débété ! !. Zanasso ! Diamankani ! TENGRELA [! ± San Koronani Kimbirila-Nord ! Toumoukoro Kanakono ! ! ! ! ! !. Ouelli Lomara Ouamélhoro Bolona ! ! Mahandiana-Sokourani Tienko ! ! B U R K I N A F A S O !. Kouban Bougou ! Blésségué ! Sokoro ! Niéllé Tahara Tiogo !. ! ! Katogo Mahalé ! ! ! Solognougo Ouara Diawala Tienny ! Tiorotiérié ! ! !. Kaouara Sananférédougou ! ! Sanhala Sandrégué Nambingué Goulia ! ! ! 10°0'0"N Tindara Minigan !. ! Kaloa !. ! M'Bengué N'dénou !. ! Ouangolodougou 10°0'0"N !. ! Tounvré Baya Fengolo ! ! Poungbé !. Kouto ! Samantiguila Kaniasso Monogo Nakélé ! ! Mamougoula ! !. !. ! Manadoun Kouroumba !.Gbon !.Kasséré Katiali ! ! ! !. Banankoro ! Landiougou Pitiengomon Doropo Dabadougou-Mafélé !. Kolia ! Tougbo Gogo ! Kimbirila Sud Nambonkaha ! ! ! ! Dembasso ! Tiasso DENGUELE REGION ! Samango ! SAVANES REGION ! ! Danoa Ngoloblasso Fononvogo ! Siansoba Taoura ! SODEFEL Varalé ! Nganon ! ! ! Madiani Niofouin Niofouin Gbéléban !. !. Village A Nyamoin !. Dabadougou Sinémentiali ! FERKESSEDOUGOU Téhini ! ! Koni ! Lafokpokaha !. Angai Tiémé ! ! [! Ouango-Fitini ! Lataha !. Village B ! !. Bodonon ! ! Seydougou ODIENNE BOUNDIALI Ponondougou Nangakaha ! ! Sokoro 1 Kokoun [! ! ! M'bengué-Bougou !. ! Séguétiélé ! Nangoukaha Balékaha /" Siempurgo ! ! Village C !. ! ! Koumbala Lingoho ! Bouko Koumbolokoro Nazinékaha Kounzié ! ! KORHOGO Nongotiénékaha Togoniéré ! Sirana -

GHANA MEGA Rockfowl & Upper Guinea Specials Th St 29 November to 21 December 2011 (23 Days)
GHANA MEGA Rockfowl & Upper Guinea Specials th st 29 November to 21 December 2011 (23 days) White-necked Rockfowl by Adam Riley Trip Report compiled by Tour Leader David Hoddinott RBT Ghana Mega Trip Report December 2011 2 Trip Summary Our record breaking trip total of 505 species in 23 days reflects the immense birding potential of this fabulous African nation. Whilst the focus of the tour was certainly the rich assemblage of Upper Guinea specialties, we did not neglect the interesting diversity of mammals. Participants were treated to an astonishing 9 Upper Guinea endemics and an array of near-endemics and rare, elusive, localized and stunning species. These included the secretive and rarely seen White-breasted Guineafowl, Ahanta Francolin, Hartlaub’s Duck, Black Stork, mantling Black Heron, Dwarf Bittern, Bat Hawk, Beaudouin’s Snake Eagle, Congo Serpent Eagle, the scarce Long-tailed Hawk, splendid Fox Kestrel, African Finfoot, Nkulengu Rail, African Crake, Forbes’s Plover, a vagrant American Golden Plover, the mesmerising Egyptian Plover, vagrant Buff-breasted Sandpiper, Four-banded Sandgrouse, Black-collared Lovebird, Great Blue Turaco, Black-throated Coucal, accipiter like Thick- billed and splendid Yellow-throated Cuckoos, Olive and Dusky Long-tailed Cuckoos (amongst 16 cuckoo species!), Fraser’s and Akun Eagle-Owls, Rufous Fishing Owl, Red-chested Owlet, Black- shouldered, Plain and Standard-winged Nightjars, Black Spinetail, Bates’s Swift, Narina Trogon, Blue-bellied Roller, Chocolate-backed and White-bellied Kingfishers, Blue-moustached, -

A Report on Birds Observed on a Trip to Cameroon 16 April – 06 May 2016
Cameroon April/May 2016 CAMEROON A report on birds observed on a trip to Cameroon 16 April – 06 May 2016 By Henk Hendriks Nyasoso village with Mount Kupe, looming in the distance Mount Cameroon Speirops Zosterops melanocephalus - Mount Cameroon 1 Cameroon April/May 2016 INTRODUCTION In October 2015 Hans Westerlaken told me that he had contacted a local guide in Cameroon who was able to organise a complete birding trip to this country for a reasonable price. He was trying to get a team of 4 birders together to undertake this trip. Knowing that Cameroon is probably the number 1 birding destination in Western Africa and having wanted to visit this country already for many years I was immediately interested. So when my brother Frans and Jan Hein van Steenis decided to join us, we had a nice team together and we could start to actually prepare ourselves for this trip. Unfortunately, because of the presence of Boko Haram in the extreme north of the country, we were unable to visit this area and had to skip for instance Waza N.P. Many of the big tour companies did not visit/ bird Cameroon in 2016 mainly because of the unrest in the Northern part of the country. Having said so, we never felt unsafe during our trip, not when we visited Ngaoundaba Ranch, Benoue N.P. and the Poli area in the north of the country either. So in the end we agreed on a 3-week itinerary which would give us the opportunity to observe most of the endemics, near-endemics and other specialties of Cameroon. -

See Full Prospectus
G20 COMPACT WITHAFRICA CÔTE D’IVOIRE Investment Opportunities G20 Compact with Africa 8°W To 7°W 6°W 5°W 4°W 3°W Bamako To MALI Sikasso CÔTE D'IVOIRE COUNTRY CONTEXT Tengrel BURKINA To Bobo Dioulasso FASO To Kampti Minignan Folon CITIES AND TOWNS 10°N é 10°N Bagoué o g DISTRICT CAPITALS a SAVANES B DENGUÉLÉ To REGION CAPITALS Batie Odienné Boundiali Ferkessedougou NATIONAL CAPITAL Korhogo K RIVERS Kabadougou o —growth m Macroeconomic stability B Poro Tchologo Bouna To o o u é MAIN ROADS Beyla To c Bounkani Bole n RAILROADS a 9°N l 9°N averaging 9% over past five years, low B a m DISTRICT BOUNDARIES a d n ZANZAN S a AUTONOMOUS DISTRICT and stable inflation, contained fiscal a B N GUINEA s Hambol s WOROBA BOUNDARIES z a i n Worodougou d M r a Dabakala Bafing a Bere REGION BOUNDARIES r deficit; sustainable debt Touba a o u VALLEE DU BANDAMA é INTERNATIONAL BOUNDARIES Séguéla Mankono Katiola Bondoukou 8°N 8°N Gontougo To To Tanda Wenchi Nzerekore Biankouma Béoumi Bouaké Tonkpi Lac de Gbêke Business friendly investment Mont Nimba Haut-Sassandra Kossou Sakassou M'Bahiakro (1,752 m) Man Vavoua Zuenoula Iffou MONTAGNES To Danane SASSANDRA- Sunyani Guemon Tiebissou Belier Agnibilékrou climate—sustained progress over the MARAHOUE Bocanda LACS Daoukro Bangolo Bouaflé 7°N 7°N Daloa YAMOUSSOUKRO Marahoue last four years as measured by Doing Duekoue o Abengourou b GHANA o YAMOUSSOUKRO Dimbokro L Sinfra Guiglo Bongouanou Indenie- Toulepleu Toumodi N'Zi Djuablin Business, Global Competitiveness, Oumé Cavally Issia Belier To Gôh CÔTE D'IVOIRE Monrovia -

C'est Nous D'abord ! Ensuite, Les Autres S'alignent…
« Chez nous, (…) c’est nous d’abord ! Ensuite, les autres s’alignent…" « Risques et Opportunités perçus pour la cohésion sociale et Gouvernance locale dans la Région du Haut-Sassandra » « Chez nous, (…) c’est nous d’abord ! Ensuite, les autres s’alignent…" « Risques et Opportunités perçus pour la cohésion sociale et Gouvernance locale dans la Région du Haut-Sassandra » Mai 2019 Cette étude a été réalisée grâce à l’appui de l’Agence Japonaise de Coopération Internationale. Le contenu de ce rapport ne reflète pas l’opinion officielle de l’Agence Japonaise de Coopération Internationale. La responsabilité des informations et points de vue exprimés dans ce dernier incombe entièrement aux personnes consultées et aux auteurs. Crédit des photos dans ce rapport : Copyright INDIGO Côte d’Ivoire Tous droits réservés. ISBN 978-2-901934-02-8 EAN 9782901934028 Copyright : Indigo Côte d’Ivoire et Interpeace 2019. Tous droits réservés. Publié en Mai 2019 Les polices typographiques utilisées dans ce rapport sont Suisse International, Suisse Works et Suisse Neue, par Swiss Typefaces qui sponsorise généreusement Interpeace. www.swisstypefaces.com Quai Perdonnet 19 1800 Vevey Switzerland La reproduction de courts extraits de ce rapport est autorisée sans autorisation écrite for- melle, à condition que la source originale soit correctement référencée, incluant le titre du rapport, l’auteur et l’année de publication. L’autorisation d’utiliser des parties de ce rapport, en entier ou en partie, peut être accordée par écrit. En aucun cas le contenu ne peut être altéré ou modifié, incluant les légendes et citations. Ceci est une publication d’Indigo Côte d’Ivoire et d’Interpeace. -

République De Cote D'ivoire
R é p u b l i q u e d e C o t e d ' I v o i r e REPUBLIQUE DE COTE D'IVOIRE C a r t e A d m i n i s t r a t i v e Carte N° ADM0001 AFRIQUE OCHA-CI 8°0'0"W 7°0'0"W 6°0'0"W 5°0'0"W 4°0'0"W 3°0'0"W Débété Papara MALI (! Zanasso Diamankani TENGRELA ! BURKINA FASO San Toumoukoro Koronani Kanakono Ouelli (! Kimbirila-Nord Lomara Ouamélhoro Bolona Mahandiana-Sokourani Tienko (! Bougou Sokoro Blésségu é Niéllé (! Tiogo Tahara Katogo Solo gnougo Mahalé Diawala Ouara (! Tiorotiérié Kaouara Tienn y Sandrégué Sanan férédougou Sanhala Nambingué Goulia N ! Tindara N " ( Kalo a " 0 0 ' M'Bengué ' Minigan ! 0 ( 0 ° (! ° 0 N'd énou 0 1 Ouangolodougou 1 SAVANES (! Fengolo Tounvré Baya Kouto Poungb é (! Nakélé Gbon Kasséré SamantiguilaKaniasso Mo nogo (! (! Mamo ugoula (! (! Banankoro Katiali Doropo Manadoun Kouroumba (! Landiougou Kolia (! Pitiengomon Tougbo Gogo Nambonkaha Dabadougou-Mafélé Tiasso Kimbirila Sud Dembasso Ngoloblasso Nganon Danoa Samango Fononvogo Varalé DENGUELE Taoura SODEFEL Siansoba Niofouin Madiani (! Téhini Nyamoin (! (! Koni Sinémentiali FERKESSEDOUGOU Angai Gbéléban Dabadougou (! ! Lafokpokaha Ouango-Fitini (! Bodonon Lataha Nangakaha Tiémé Villag e BSokoro 1 (! BOUNDIALI Ponond ougou Siemp urgo Koumbala ! M'b engué-Bougou (! Seydougou ODIENNE Kokoun Séguétiélé Balékaha (! Villag e C ! Nangou kaha Togoniéré Bouko Kounzié Lingoho Koumbolokoro KORHOGO Nongotiénékaha Koulokaha Pign on ! Nazinékaha Sikolo Diogo Sirana Ouazomon Noguirdo uo Panzaran i Foro Dokaha Pouan Loyérikaha Karakoro Kagbolodougou Odia Dasso ungboho (! Séguélon Tioroniaradougou -

Impact of Covid-19 on Food Security in the Sub-Prefecture of Vavoua (Côte D'ivoire)
Quest Journals Journal of Research in Humanities and Social Science Volume 9 ~ Issue 6 (2021)pp: 51-58 ISSN(Online):2321-9467 www.questjournals.org Research Paper Impact of covid-19 on food security in the sub-prefecture of vavoua (Côte d'Ivoire) KRA Koffi Siméon Teacher-Researcher, Jean Lorougnon Guédé University of Daloa KOFFI Bouadi Arnaud Ferrand Teacher-Researcher, Jean Lorougnon Guédé University of Daloa Corresponding Author: KOFFI Bouadi Arnaud ABSTRACT: Located in the central-western part of Côte d'Ivoire, the sub-prefecture of Vavoua, like other rural localities in the country, has been suffering the consequences of COVID-19 since 11th March 2020, the date of the first case in Côte d'Ivoire. In response to the shock created by this disease, several measures have been taken by the government, including the Emergency Support Programme for Agricultural Industries Impacted by Covid-19 (PURGA). The objective of this article is to show the impact of this programme on the food security of the beneficiary populations of the sub-prefecture of Vavoua in a Covid-19 context. To achieve this, the methodology adopted is based on documentary research, interview guides and a questionnaire survey. Our study shows that PURGA has contributed to the food security of beneficiary populations in the sub- prefecture of Vavoua. Indeed, PURGA has contributed to the availability and accessibility food products exclusively used for human consumption, both physically and economically. KEYWORDS:Côte d'Ivoire, Vavoua, COVID-19, impact, food security Received 14 June, 2021; Revised: 27 June, 2021; Accepted 29 June, 2021 © The author(s) 2021.