CURRICULUM VITAE JUDITH CAMPISI Buck Institute For
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

University Medical Center of the University of Groningen Research Portfolio
University Medical Center of the University of Groningen Research portfolio Research Institutes and Research Programmes UMCG’s multidisciplinary Research Programmes are organised in five Research Institutes. Each Institute covers a specific part of the UMCG research area. By establishing numerous international research projects and strategic alliances over the past, research within the Institutes bridged national boundaries. Especially for the UMCG focus "Active and Healty Ageing" international collaboration is required and prepares the UMCG to contribute to this global challenge. For every UMCG Research Programme the relevance for Healthy Ageing is defined. Regarding the training and education of future scientists (MSc and PhD) the five Research Institutes collaborate in the Graduate School of Medical Sciences assuring the incorporation of state of the art scientific know-how. 1. GUIDE Institute: Chronic Diseases and Drug Exploration 2. BCN-BRAIN Institute: Behavioural and Cognitive Neurosciences 3. SHARE Institute: Health Research and Epidemiology 4. W.J.Kolff Institute: Biomaterials 5. CRCG Institute: Fundamental, Clinical and Translational Cancer Research Some of the Research Programmes are Platform Programmes, embedded in and supporting all five Research Institutes. Contents: Research programmes GUIDE 02-26 Research programmes BCN-BRAIN 27-35 Research programmes SHARE 36-46 Research programmes CRCG 47-55 Research programmes W.J. Kolff 56-65 Platform programme Center Medical Imaging 66-68 1 RESEARCH INSTITUTE GUIDE: Chronic Diseases and Drug Exploration 1. Biopharmaceuticals, Discovery, Design and Delivery (GUIDE-BDDD) Programme leaders: prof. dr. H.W. Frijlink, prof. dr. K. Poelstra Mission The BDDD Division explores innovative approaches oriented towards the early phase of drug development up to the use of these approaches in practice. -

Tuesday 24Th
Matchmaking event University of Groningen/UMCG and University of Chile 24-25 September, 2019 Time slot Tuesday 24th Location 09:00-10:30 Health Kick-off ERIBA Seminar Room 10:30-11:00 Break ERIBA Pantry 11:00-13:00 Parallel session Time Neuroscience & Biology of ageing (ERIBA seminar room) Time Oncology & Drug Delivery (Room 16) 11:00-11:25 Felipe Court: 11:00-11:20 Andrew Quest: Necroaxoptosis: a novel axonal degenerative mechanism involved in pathologies of the ageing nervous system From tumor suppressor to metastasis promoter – Caveolin-1, a Jack-of-all trades in cancer 11:25-11:40 Marco Demaria: 11:20-11:40 Frank Kruyt: Role of cellular senescence in health and disease Brain tumors: glioblastoma stem cell models and identification of new therapeutic targets 11:40-12:02 Miguel Concha: 11:40-12:00 Marcelo Kogan: Nothobranchious furzeru as a model of aging and neurodegeneration Nanoplatforms for drug delivery, theraphy and diagnostic of chronic diseases 12:05-12:20 Harrie Kampinga: 12:00-12:20 Paul de Vos: Protein homeostasis and age-related protein aggregation diseases Carbohydrates and microbiota in health 12:20-12:45 Christian González-Billault: 12:20-12:40 Inge Zuhorn: Understanding neuronal aging using cell culture models Drug Delivery across the Blood-Brain Barrier 12:45-13:00 Amalia Dolga: 12:40-13:00 Wijnand Helfrich: Targeting mitochondria in human neurodegenerative disease model systems Novel Targeted approaches in Cancer Immunotherapy 13-14:30 Lunch ERIBA Pantry 15:00-17:30 Parallel session Time Neuroscience & Biology of ageing -

Cellular Senescence Promotes Skin Carcinogenesis Through P38mapk and P44/P42mapk Signaling
Author Manuscript Published OnlineFirst on July 8, 2020; DOI: 10.1158/0008-5472.CAN-20-0108 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. Cellular senescence promotes skin carcinogenesis through p38MAPK and p44/p42 MAPK signaling Fatouma Alimirah1, Tanya Pulido1, Alexis Valdovinos1, Sena Alptekin1,2, Emily Chang1, Elijah Jones1, Diego A. Diaz1, Jose Flores1, Michael C. Velarde1,3, Marco Demaria1,4, Albert R. Davalos1, Christopher D. Wiley1, Chandani Limbad1, Pierre-Yves Desprez1, and Judith Campisi1,5* 1Buck Institute for Research on Aging, Novato, CA 94945, USA 2Dokuz Eylul University School of Medicine, Izmir 35340, Turkey 3Institute of Biology, University of the Philippines Diliman, College of Science, Quezon City 1101, Philippines 4European Research Institute for the Biology of Ageing, University Medical Center Groningen, Groningen, the Netherlands 5Biosciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA 94720, USA *Lead Contact: Judith Campisi, Buck Institute for Research on Aging, 8001 Redwood Boulevard, Novato, CA 94945, USA; Email: [email protected] or [email protected]. Telephone: 1-415-209-2066; Fax: 1-415-493-3640 Running title: Senescence and skin carcinogenesis Key words: doxorubicin, senescence-associated secretory phenotype, squamous cell carcinoma, microenvironment, inflammation, transgenic mice, tumorspheres Conflict of Interests JC is a co-founder of Unity Biotechnology and MD owns equity in Unity Biotechnology. All other authors declare no competing financial interests. Downloaded from cancerres.aacrjournals.org on September 25, 2021. © 2020 American Association for Cancer Research. Author Manuscript Published OnlineFirst on July 8, 2020; DOI: 10.1158/0008-5472.CAN-20-0108 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. -

SENS-Research-Foundation-2019
by the year 2050, cardiovascular an estimated 25-30 the american 85 percent of adults disease years and older age 85 or older remains the most population will suffer from common cause of 2 1 2 dementia. death in older adults. triple. THE CLOCK IS TICKING. By 2030, annual direct The estimated cost of medical costs associated dementia worldwide was 62% of Americans with cardiovascular $818 billion diseases in the united over age 65 have in 2015 and is states are expected to more than one expected to grow to rise to more than chronic condition.1 3 $2 trillion $818 billion. by 2030.1 References: (1) https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5732407/, (2) https://www.who.int/ageing/publications/global_health.pdf, (3) https://www.cdcfoundation.org/pr/2015/heart-disease-and-stroke-cost-america-nearly-1-billion-day-medical-costs-lost-productivity sens research foundation board of directors Barbara Logan Kevin Perrott Bill Liao Chairperson Treasurer Secretary Michael Boocher Kevin Dewalt James O’Neill Jonathan Cain Michael Kope Frank Schuler 02 CONTENTS 2019 Annual Report 04 Letter From The CEO 06 Outreach & Fundraising 08 Finances 09 Donors erin ashford photography 14 Education 26 Investments 20 Conferences & Events 30 Research Advisory Board 23 Speaking Engagements 31 10 Years Of Research 24 Alliance 32 MitoSENS 34 LysoSENS 35 Extramural Research 38 Publications 39 Ways to Donate cover Photo (c) Mikhail Leonov - stock.adobe.com special 10th anniversary edition 03 FROM THE CEO It’s early 2009, and it’s very late at night. Aubrey, Jeff, Sarah, Kevin, and Mike are sitting around a large table covered in papers and half-empty food containers. -

Annual Report V2.Indd 1 16-07-2020 20:59 Welcome to Our Annual Report 2019
European Research Institute for the Biology of Ageing Annual Report 2019 ERIBA | Cover .indd Alle pagina's 30-06-2020 13:49 Annual Report 2019 2019 ERIBA | Annual Report V2.indd 1 16-07-2020 20:59 Welcome to our Annual Report 2019 Coordination: Gerald de Haan and Megha Upadhyay Secretarial Support: Sylvia Hoks, Annet Vos-Hassing & Alida de Haan ژيƺɀǣǕȇƏȇƳXǼǼɖɀɎȸƏɎǣȒȇɀ) Stefan Heinrich ژيȸǣȇɎǣȇǕ¨ Ridderprint BV | Anand Baldew Copies: 100 ERIBA | Annual Report V2.indd 2 16-07-2020 20:59 Annual Report Table of contents 1. Foreword by the Director 4 2. Ageing Research at ERIBA 6 3. 2019: Highlights 10 4.Facts and Figures 20 אא ³ƬǣƺȇɎǣˡƬ¨ɖƫǼǣƬƏɎǣȒȇɀٮ -Funding/Grants 32 -Invited Speakers 35 אג ƺȒȵǼƺ¨ٮ 5.Facilities 46 6.Education 50 7.Outreach & Dissemination 54 זד ³ƬǣƺȇɎǣˡƬƳɮǣɀȒȸɵ ȒƏȸƳِז 9.Sponsors 59 ERIBA | Annual Report V2.indd 3 16-07-2020 20:59 Foreword by the Director 2019 in review It is a great pleasure to present to you the 2019 Annual Report of the European Research Institute for the Biology of Ageing. This report provides you with an overview of all our activities and achievements, in science, education, business development and outreach. We value all these domains equally, and are proud to share with you all that has been accomplished in 2019. I write these words in the midst of the Covid-19 pandemic. This pandemic may very well have a major effect on global research and education for quite some time to come. One aspect, highly relevant to what we do in ERIBA, relates to how the virus differentially affects individuals in society. -

Shared Ageing Research Models (Sharm): a New Facility to Support Ageing Research
Biogerontology (2013) 14:789–794 DOI 10.1007/s10522-013-9457-0 METHOD Shared Ageing Research Models (ShARM): a new facility to support ageing research Adele L. Duran • Paul Potter • Sara Wells • Tom Kirkwood • Thomas von Zglinicki • Anne McArdle • Cheryl Scudamore • Qing-Jun Meng • Gerald de Haan • Anne Corcoran • Ilaria Bellantuono Received: 5 July 2013 / Accepted: 16 August 2013 / Published online: 2 October 2013 Ó The Author(s) 2013. This article is published with open access at Springerlink.com Abstract In order to manage the rise in life expec- Wellcome Trust, open to all investigators. It collects, tancy and the concomitant increased occurrence of stores and distributes flash frozen tissues from aged age-related diseases, research into ageing has become murine models through its biorepository and provides a strategic priority. Mouse models are commonly a database of live ageing mouse colonies available in utilised as they share high homology with humans and the UK and abroad. It also has an online environment show many similar signs and diseases of ageing. (MICEspace) for collation and analysis of data from However, the time and cost needed to rear aged communal models and discussion boards on subjects cohorts can limit research opportunities. Sharing of such as the welfare of ageing animals and common resources can provide an ethically and economically endpoints for intervention studies. Since launching in superior framework to overcome some of these issues July 2012, thanks to the generosity of researchers in but requires dedicated infrastructure. Shared Ageing UK and Europe, ShARM has collected more than Research Models (ShARM) (www.ShARMUK.org) 2,500 tissues and has in excess of 2,000 mice regis- is a new, not-for-profit organisation funded by tered in live ageing colonies. -
Viewer Comments
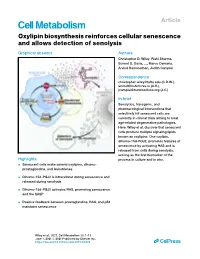
Article Oxylipin biosynthesis reinforces cellular senescence and allows detection of senolysis Graphical abstract Authors Christopher D. Wiley, Rishi Sharma, Sonnet S. Davis, ..., Marco Demaria, Arvind Ramanathan, Judith Campisi Correspondence [email protected] (C.D.W.), [email protected] (A.R.), [email protected] (J.C.) In brief Senolytics, transgenic, and pharmacological interventions that selectively kill senescent cells are currently in clinical trials aiming to treat age-related degenerative pathologies. Here, Wiley et al. discover that senescent cells produce multiple signaling lipids known as oxylipins. One oxylipin, dihomo-15d-PGJ2, promotes features of senescence by activating RAS and is released from cells during senolysis, serving as the first biomarker of the Highlights process in culture and in vivo. d Senescent cells make several oxylipins, dihomo- prostaglandins, and leukotrienes d Dihomo-15d-PGJ2 is intracellular during senescence and released during senolysis d Dihomo-15d-PGJ2 activates RAS, promoting senescence and the SASP d Positive feedback between prostaglandins, RAS, and p53 maintains senescence Wiley et al., 2021, Cell Metabolism 33, 1–13 June 1, 2021 ª 2021 Published by Elsevier Inc. https://doi.org/10.1016/j.cmet.2021.03.008 ll Please cite this article in press as: Wiley et al., Oxylipin biosynthesis reinforces cellular senescence and allows detection of senolysis, Cell Metabolism (2021), https://doi.org/10.1016/j.cmet.2021.03.008 ll Article Oxylipin biosynthesis reinforces cellular senescence and allows detection of senolysis Christopher D. Wiley,1,2,* Rishi Sharma,1 Sonnet S. Davis,1 Jose Alberto Lopez-Dominguez,1 Kylie P. Mitchell,1 Samantha Wiley,1 Fatouma Alimirah,1 Dong Eun Kim,1 Therese Payne,1 Andrew Rosko,1 Eliezer Aimontche,1 Sharvari M. -

Longevity, Aging, and Caloric Restriction : Clive Maine Mccay and the Construction of a Multidisciplinary Research Program
This document is downloaded from DR‑NTU (https://dr.ntu.edu.sg) Nanyang Technological University, Singapore. Longevity, aging, and caloric restriction : Clive Maine McCay and the construction of a multidisciplinary research program Park, Hyung Wook 2010 Park, H. W. (2010). Longevity, Aging, and Caloric Restriction: Clive Maine McCay and the Construction of a Multidisciplinary Research Program. Historical Studies in the Natural Sciences, 40(1), 79‑124. https://hdl.handle.net/10356/96440 https://doi.org/10.1525/hsns.2010.40.1.79 © 2010 by the Regents of the University of California. This paper was published in Historical Studies in the Natural Sciences and is made available as an electronic reprint (preprint) with permission of the Regents of the University of California. The paper can be found at the following official DOI: [http://dx.doi.org/10.1525/hsns.2010.40.1.79]. One print or electronic copy may be made for personal use only. Systematic or multiple reproduction, distribution to multiple locations via electronic or other means, duplication of any material in this paper for a fee or for commercial purposes, or modification of the content of the paper is prohibited and is subject to penalties under law. Downloaded on 02 Oct 2021 12:53:04 SGT HYUNG WOOK PARK* Longevity, Aging, and Caloric Restriction: Clive Maine McCay and the Construction of a Multidisciplinary Research Program ABSTRACT Since the 1930s scientists from fields such as biochemistry, pathology, immunology, genetics, neuroscience, and nutrition have studied the relation of dietary caloric intake to longevity and aging. This paper discusses how Clive Maine McCay, a professor of animal husbandry at Cornell University, began his investigation of the topic and pro- moted it as a productive research program in the multidisciplinary science of geron- tology. -

Cellular Senescence and Apoptosis: How Cellular Responses Might Influence Aging Phenotypes
Experimental Gerontology 38 (2003) 5–11 www.elsevier.com/locate/expgero Cellular senescence and apoptosis: how cellular responses might influence aging phenotypes Judith Campisia,b,* aLife Sciences Division, Lawrence Berkeley National Laboratory, 1 Cyclotron Road, Berkeley, CA 94720, USA bBuck Institute for Age Research, 8001 Redwood Blvd., Novato, CA 94945, USA Received 20 May 2002; accepted 28 May 2002 Abstract Aging in complex multi-cellular organisms such as mammals entails distinctive changes in cells and molecules that ultimately compromise the fitness of adult organisms. These cellular and molecular changes lead to the phenotypes we recognize as aging. This review discusses some of the cellular and molecular changes that occur with age, specifically changes that occur as a result of cellular responses that evolved to ameliorate the inevitable damage that is caused by endogenous and environmental insults. Because the force of natural selection declines with age, it is likely that these processes were never optimized during their evolution to benefit old organisms. That is, some age- related changes may be the result of gene activities that were selected for their beneficial effects in young organisms, but the same gene activities may have unselected, deleterious effects in old organisms, a phenomenon termed antagonistic pleiotropy. Two cellular processes, apoptosis and cellular senescence, may be examples of antagonistic pleiotropy. Both processes are essential for the viability and fitness of young organisms, but may contribute to aging phenotypes, including certain age-related diseases. q 2002 Elsevier Science Inc. All rights reserved. Keywords: Antagonistic pleiotropy; Apoptosis; Cancer; Cellular senescence; DNA damage response; Neurodegeneration; p53; Telomeres 1. -

Incarnation and the Dynamics of Medical Promises: DHEA As a Fountain of Youth Hormone Boris Hauray, Sébastien Dalgalarrondo
Incarnation and the dynamics of medical promises: DHEA as a fountain of youth hormone Boris Hauray, Sébastien Dalgalarrondo To cite this version: Boris Hauray, Sébastien Dalgalarrondo. Incarnation and the dynamics of medical promises: DHEA as a fountain of youth hormone. Health, SAGE Publications, 2018, 23 (6), pp.639-655. 10.1177/1363459318769437. halshs-02437749 HAL Id: halshs-02437749 https://halshs.archives-ouvertes.fr/halshs-02437749 Submitted on 13 Jan 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Incarnation and the dynamics of medical promises: DHEA as a fountain of youth hormone Boris Hauray, Sébastien Dalgalarrondo To cite this version: Boris Hauray, Sébastien Dalgalarrondo. Incarnation and the dynamics of medical promises: DHEA as a fountain of youth hormone. Health, SAGE Publications, 2018, 23 (6), pp.639-655. 10.1177/1363459318769437. halshs-02437749 HAL Id: halshs-02437749 https://halshs.archives-ouvertes.fr/halshs-02437749 Submitted on 13 Jan 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. -

Searching for SETI: the Social Construction of Aliens and the Quest for a Technological Mythos
Searching for SETI: The Social Construction of Aliens and the Quest for a Technological Mythos John Marvin Bozeman Dissertation submitted to the faculty of the Virginia Polytechnic Institute and State University in partial fulfillment of the requirements for the degree of Doctor of Philosophy In Science and Technology Studies Janet A. Abbate, Co-Chair Shannon A. Brown, Co-Chair Lee L. Zwanziger Paul D. Renard March 24, 2015 Falls Church, Virginia Keywords: Search for Extraterrestrial Intelligence, SETI, Actor Network Theory, Social Construction of Technology, Rational Choice Theory of Religion, Transhumanism, Xenosalvation Copyright John M. Bozeman Searching for SETI: The Social Construction of Aliens and the Quest for a Technological Mythos John M. Bozeman ABSTRACT This dissertation uses Actor Network Theory (ANT) and Stark and Bainbridge’s rational choice theory of religion to analyze an established but controversial branch of science and technology, the Search for Extraterrestrial Intelligence (SETI). Of particular interest are the cultural, and sometimes religious, assumptions that its creators have built into it. The purpose of this analysis is not to discredit SETI, but instead to show how SETI, along with other avant-garde scientific projects, is founded, motivated, and propelled by many of the same types of values and visions for the future that motivate the founders of religious groups. I further argue that the utopian zeal found in SETI and similar movements is not aberrant, but instead common, and perhaps necessary, in many early- stage projects, whether technical or spiritual, which lack a clear near-term commercial or social benefit. DEDICATION In memory of my parents, James E. -

Cellular Senescence and the Senescent Secretory Phenotype: Therapeutic Opportunities
Cellular senescence and the senescent secretory phenotype: therapeutic opportunities Tamara Tchkonia, … , Judith Campisi, James L. Kirkland J Clin Invest. 2013;123(3):966-972. https://doi.org/10.1172/JCI64098. Review Series Aging is the largest risk factor for most chronic diseases, which account for the majority of morbidity and health care expenditures in developed nations. New findings suggest that aging is a modifiable risk factor, and it may be feasible to delay age-related diseases as a group by modulating fundamental aging mechanisms. One such mechanism is cellular senescence, which can cause chronic inflammation through the senescence-associated secretory phenotype (SASP). We review the mechanisms that induce senescence and the SASP, their associations with chronic disease and frailty, therapeutic opportunities based on targeting senescent cells and the SASP, and potential paths to developing clinical interventions. Find the latest version: https://jci.me/64098/pdf Review series Cellular senescence and the senescent secretory phenotype: therapeutic opportunities Tamara Tchkonia,1 Yi Zhu,1 Jan van Deursen,1 Judith Campisi,2 and James L. Kirkland1 1Robert and Arlene Kogod Center on Aging, Mayo Clinic, Rochester, Minnesota, USA. 2Buck Institute for Research on Aging, Novato, California, USA. Aging is the largest risk factor for most chronic diseases, which account for the majority of morbidity and health care expenditures in developed nations. New findings suggest that aging is a modifiable risk factor, and it may be feasible to delay age-related diseases as a group by modulating fundamental aging mechanisms. One such mech- anism is cellular senescence, which can cause chronic inflammation through the senescence-associated secretory phenotype (SASP).