The Impact of Ph on the Structure and Function of Neural Cadherin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Kémia Idegen Nyelven 147
Kémia idegen nyelven 147 KÉMIA IDEGEN NYELVEN Kémia németül Szerkesztő: Horváth Judit A 2014/4. számban megjelent szakszöveg helyes fordítása: Pigmentek és festékek1 Pigmentek és kötőanyagok2 A pigment a felhasználásra szánt közegben (lakk, festék) oldhatatlan festékanyag, mely legtöbbször nagyon finoman eloszlatott formában van jelen. Az oldható festékanyagokat ezzel szemben színezékeknek3 színezékeket inkább színezésre. A tiszta pigment nem tartalmaz adaléknevezzük.anyago Pigmenteketkat. főként festésnél hatása és mázolásnál a kémiai összetételhasználunk, 4 függ. Az optimális Egyrészecskeméret pigment színező egy ötszázad és egy kétezredtől, millimétera kristályszerkezettől, között van. a részecskemérettől és az egész belső felülettől Ahhoz, hogy egy festékpigment tapadjon egy felületre, kötőanyaggal kell összekeverni. A kötőanyagok olyan anyagok, melyek finom eloszlású egyéb anyagokat felragasztanak egy alapra. Festék megszilárdulása alapon 5. Felhasználásielőállításához aterület pigmentet és festékkötőanyaggal6 keverik össze, és annak alkalmatosak. Használatután a pigment a festendő rögzítődik fehérjék, a szerint különféle kötőanyagok lenolaj hozzákeverésévelosak a gumik, olajfestéket. gyanták, Aviaszok, vízfestékekben olajok, cellulóz vagy a mész. Így kapunk például az ultramarinkék pigmentből Az iskolákban gyakran használt gumiarábikumot, a kazeinfestékekben a kazein nevű tejfehérjét alkalmazzák kötőanyagnak. 148 Kémia idegen nyelven plakátfesték7 a Pelikan temperafesték enyv–olaj–víz emulziót tartalmaz a pigment megkötésére. Az -

A Comprehensive Self-Consistent Spectrophotometric Acidity Scale Of
A Comprehensive Self-Consistent Spectrophotometric Acidity Scale of Neutral Brønsted Acids in Acetonitrile Agnes Kütt †, Ivo Leito * †, Ivari Kaljurand †, Lilli Sooväli †, Vladislav M. Vlasov ‡, Lev M. Yagupolskii § and Ilmar A. Koppel † † Institute of Chemical Physics, Department of Chemistry, University of Tartu, Jakobi 2 Str., 51014 Tartu, Estonia. E-mail: [email protected]; Phone: +372 7 375 259; Fax: +372 7 375 264. ‡ Institute of Organic Chemistry, Siberian Branch of the Russian Academy of Sciences, Novosibirsk 630090, Russia § Institute of Organic Chemistry, Ukrainian National Academy of Sciences, Murmanskaya Str. 5, Kiev 02094, Ukraine. Abstract For the first time the self-consistent spectrophotometric acidity scale of neutral Brønsted acids in acetonitrile (AN) spanning 24 orders of magnitude of acidities is reported. The scale ranges from pKa value 3.7 to 28.1 in AN. The scale includes altogether 93 acids that are interconnected by 203 relative acidity measurements (∆pKa measurements). The scale 1 contains compounds with gradually changing acidities, including representatives from all the conventional families of OH (alcohols, phenols, carboxylic acids, sulfonic acids), NH (anilines, diphenylamines, disulfonimides) and CH acids (fluorenes, diphenylacetonitriles, phenylmalononitriles). The CH acids were particularly useful in constructing the scale because they do not undergo homo- or heteroconjugation processes and their acidities are rather insensitive to traces of water in the medium. The scale has been fully cross-validated: the relative acidity of any two acids on the scale can be found combining at least two independent sets of ∆pKa measurements. The consistency standard deviation of the scale is 0.03 pKa units. Comparison of acidities in many different media has been carried out and the structure-acidity relations are discussed. -
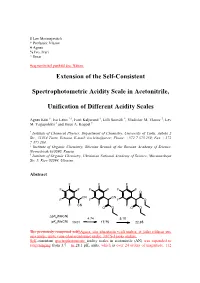
Extension of the Self-Consistent Spectrophotometric Acidity Scale In
$ Lev Moissejevitch * Professor Vlasov # Agnes % Ivo, Ivari ^ Ilmar #eq numbritel punktid ära. Näitan. Extension of the Self-Consistent Spectrophotometric Acidity Scale in Acetonitrile, Unification of Different Acidity Scales Agnes Kütt †, Ivo Leito *,†, Ivari Kaljurand †, Lilli Sooväli †, Vladislav M. Vlasov ‡, Lev M. Yagupolskii § and Ilmar A. Koppel † † Institute of Chemical Physics, Department of Chemistry, University of Tartu, Jakobi 2 Str., 51014 Tartu, Estonia. E-mail: [email protected]; Phone: +372 7 375 259; Fax: +372 7 375 264. ‡ Institute of Organic Chemistry, Siberian Branch of the Russian Academy of Science, Novosibirsk 630090, Russia § Institute of Organic Chemistry, Ukrainian National Academy of Science, Murmanskaya Str. 5, Kiev 02094, Ukraine. Abstract F F F F F F F F F O CN CN F F F O F CN F F O O O O ∆pK (MeCN) a 4.74 5.10 pK (MeCN) a 13.01 17.75 22.85 The previously composed self#Agnes, siin sõnastasin veidi umber, et jääks rohkem uue asja mulje, mitte vana edasiarendamise mulje. JACS-I jaoks oluline. Self-consistent spectrophotometric acidity scales in acetonitrile (AN) was expanded to rangeranging from 3.7 – to 28.1 pKa units, which is over 24 orders of magnitude. 112 newreported. Altogether 203 acidity measurements werehave been carried out and 49 new compounds were93 acids are included to in the scale. With previous scales altogether 95 compounds are included to the present scale. The relative acidity (∆pKa value) of any two acids on the scale can be obtained by combainingcombining at least two independent sets of measurements. This multiple overlapping measurements makes results more reliableof measurements is essential to achieve high reliability of the pKa values. -

KARL KAUPMEES Acidity and Basicity in Non-Aqueous Media
DISSERTATIONES CHIMICAE KARL KAUPMEES UNIVERSITATIS TARTUENSIS 141 Acidity and basicity in non-aqueous media: importance of solvent properties and purity importanceproperties of solvent Acidity and basicity in non-aqueous media: KARL KAUPMEES Acidity and basicity in non-aqueous media: importance of solvent properties and purity Tartu 2014 ISSN 1406–0299 ISBN 978-9949-32-635-8 DISSERTATIONES CHIMICAE UNIVERSITATIS TARTUENSIS 141 DISSERTATIONES CHIMICAE UNIVERSITATIS TARTUENSIS 141 KARL KAUPMEES Acidity and basicity in non-aqueous media: importance of solvent properties and purity Institute of Chemistry, Faculty of Science and Technology, University of Tartu. Dissertation is accepted for the commencement of the Degree of Doctor philo- sophiae in Chemistry on June 17, 2014 by the Doctoral Committee of the Institute of Chemistry, University of Tartu. Supervisor: Professor Ivo Leito (PhD) Senior research fellow Ivari Kaljurand (PhD) Institute of Chemistry, University of Tartu, Estonia Opponent: Prof. José Luis Abboud Mas (D.Sc.) Retired professor, Instituto de Química Física “Rocasolano”, CSIC, Madrid, Spain Commencement: August 29, 2014 at 12:00, Ravila 14a, room 1021 This work has been partially supported by Graduate School “Functional materials and technologies” receiving funding from the European Social Fund under project 1.2.0401.09-0079 in University of Tartu, Estonia. Publication of this dissertation is granted by University of Tartu ISSN 1406-0299 ISBN 978-9949-32-635-8 (print) ISBN 978-9949-32-636-5 (pdf) Copyright: Karl Kaupmees, -

Synthesis and Characterization of Ammonium Ionenes Containing Hydrogen Bonding Functionalities
Synthesis and Characterization of Ammonium Ionenes Containing Hydrogen Bonding Functionalities Mana Tamami Dissertation submitted to the faculty of the Virginia Polytechnic Institute and State University in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Chemistry Timothy E. Long, Chair Garth L. Wilkes Robert B. Moore S. Richard Turner Susan E. Duncan December 6, 2012 Blacksburg, Virginia Keywords: Segmented ionenes, non-segmented ionenes, nucleobase, hydrogen bonding, Michael addition, self-healing, bio-adhesion, molecular recognition, step-growth polymerization, non- covalent interactions Copyright © 2012, Mana Tamami iii Synthesis and Characterization of Ammonium Ionenes Containing Hydrogen Bonding Functionalities Mana Tamami Abstract Ammonium ionenes are polycations that have quaternary nitrogens in their macromolecular backbone and are synthesized via step-growth polymerization technique. They offer interesting coulombic properties, and the synthetic design provides control over charge density. Non- covalent interactions including nucleobase hydrogen bonding and electrostatics were studied in ammonium ionenes. The non-covalent interactions are expected to increase the effective molecular weight of polymeric precursors and induce microphase separation due to intermolecular associations. The influence of non-covalent interactions on structure-property relationships of ammonium ionenes were studied regarding mechanical (tensile, DMA), thermal (DSC, TGA), and morphological (AFM, SAXS) properties. Hydrogen bonding interaction (10-40 kJ/mol) was introduced using DNA nucleobase pairs such as adenine and thymine. Novel adenine and thymine functionalized segmented and non- segmented ammonium ionenes were successfully synthesized using Michael addition chemistry. In non-segmented systems, we investigated the influence of spacer length on homoassociation and heteroassociation of complementary nucleobase-containing ionenes. Based on DSC analyses, complementary non-segmented ionenes made miscible blends. -

Unexpected Solvation-Stabilisation of Ions in a Protic Ionic Liquid: Insights Disclosed by a Bond Cite This: Chem
Chemical Science View Article Online EDGE ARTICLE View Journal | View Issue Unexpected solvation-stabilisation of ions in a protic ionic liquid: insights disclosed by a bond Cite this: Chem. Sci.,2018,9, 3538 energetic study† Zhen Wang,ac Feixiang Gao,a Pengju Ji *a and Jin-Pei Cheng *ab Equilibrium acidities (pKas) of 42 organic acids were precisely determined in protic ionic liquid (PIL) [DBUH] [OTf]. Surprisingly, the often seen homoassociation complication during the pKa measurement of O–H acids in DMSO was not detected in [DBUH][OTf], implying that the incipient oxanion should be better solvation-stabilized by the PIL, although its “apparent” dielectric constant is much lower than that of the conventional molecular solvent DMSO. Evidence showing that the solute ions in the PIL are also free from other specific ion associations like ion-pairing is further demonstrated by the identical pKasof protic amine salts bearing largely different counter-anions. Correlations between the RO–H, N–H, N+–H Received 9th December 2017 and RCOO–H bond acidities in [DBUH][OTf] and in water revealed different slopes and intercepts for Accepted 5th March 2018 + Creative Commons Attribution-NonCommercial 3.0 Unported Licence. each individual series, suggesting far superior properties of the DBUH -based PIL for differentiating the DOI: 10.1039/c7sc05227h solvation effect of various species in structural analysis to the well applied EAN that is known for leveling rsc.li/chemical-science out differential solvation. Introduction ionicity degree and solvation behaviours, -

Retention-Ph Profiles of Acids and Bases in Hydrophilic Interaction Liquid Chromatography
Retention-pH profiles of acids and bases in hydrophilic interaction liquid chromatography Tamara Alvarez-Segura1,2, Xavier Subirats1, Martí Rosés1,* 1Institute of Biomedicine (IBUB) and Department of Chemical Engineering and Analytical Chemistry, Universitat de Barcelona, Martí i Franquès 1-11, 08028 Barcelona, Spain 2Department of Analytical Chemistry, University of Valencia, Dr. Moliner 50, 46100 Burjassot (Valencia), Spain *Corresponding author Ms. Tamara Alvarez-Segura E-mail: [email protected] Dr. Xavier Subirats Phone: +34 934 039 119, Fax: +34 934 021 233, E-mail: [email protected] Prof. Martí Rosés Phone: +34 934 039 275, Fax: +34 934 021 233, E-mail: [email protected] 1 Abstract The high proportion of acetonitrile used in many HILIC mobile phases significantly changes the acid-base properties of pH buffers and analytes foreseen from available data in water. In this paper, the recommended stability pH range for chromatographic columns is examined with various acetonitrile water mixtures, resulting in a significant broadening in the operational pH window with the content of organic solvent. Additionally, the challenge of buffer selection in HILIC is also addressed. Commonly used ammonium acetate shrinks its pH buffering range in acetonitrile-rich mobile phases due to variations in the dissociation constants of the buffer constituents (acetic acid and ammonium). Thus, other organic acids such as formic acid, TFA, and succinimide have been studied as buffers in order to fully cover the pH range of use of the column. Also the retention-pH profiles of several acids and bases have been studied in 80% and 90% acetonitrile using the proposed buffers and their behavior compared to that obtained with buffers prepared from oxalic acid, pyrrolidine, and triethylamine. -

GLOSSARY of TERMS USED in PHYSICAL ORGANIC CHEMISTRY (IUPAC Recommendations 1994)
Pure &Appl. Chem., Vol. 66, No. 5, pp. 1077-1184,1994. Printed in Great Britain. 0 1994 IUPAC INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY ORGANIC CHEMISTRY DIVISION COMMISSION ON PHYSICAL ORGANIC CHEMISTRY* GLOSSARY OF TERMS USED IN PHYSICAL ORGANIC CHEMISTRY (IUPAC Recommendations 1994) Prepared for publication by P. MULLER DCpartement de Chimie Organique, UniversitC de Genbve, CH-1211 Genbve 4, Suisse *Membership of the Commission during the period (1987-93) the report was drafted was as follows: Chairman: 1987-93 P. Muller (Switzerland); Secretary: 1987-91 M. P. Doyle (USA); 1991-93 W. Drenth (Netherlands). Titular Members: P. N. I. Ahlberg (Sweden, 1987-91); E. A. Halevi (Israel, 1987-89); J. M. McBride (USA, 1987-93); V. Minkin (USSR, 1991-93); 0. M. Nefedov (USSR, 1987-91); M. Oki (Japan, 1987-91); J. Shorter (UK, 1989-93); Y. Takeuchi (Japan, 1991-93); Z. Rappoport (Israel, 1991-93); Associate Members: P. N. I. Ahlberg (Sweden, 1991-93); T. A. Albright (USA, 1987-91); W. Drenth (Netherlands, 1987-91); E. A. Halevi (Israel, 1989-93); R. A. Y. Jones (UK, 1987-89); V. I. Minkin (USSR, 1987-91); 0. M. Nefedov (USSR, 1991-93); C. Pemn (USA, 1991-93); D. Raber (USA, 1991-93); K. Schwetlick (GDR, 1987-89); Y. Takeuchi (Japan, 1987-91); P. Van Brandt (Belgium, 1987-93); J. R. Zdysiewicz (Australia, 1989-93); National Representatives: J.-L. Abboud Mas (Spain, 1991-93); E. Baciocchi (Italy, 1989-93); M. V. Bhatt (India, 1989-91); X. Jiang (China, 1987-93); J. J. E. Humeres Allende (Brazil, 1991-93); P. Laszlo (Belgium, 1987-91); D. -

Supplementary Information
Electronic Supplementary Material (ESI) for Chemical Science. This journal is © The Royal Society of Chemistry 2018 Supplementary Information Unexpected Solvation-stabilization of Ions in Protic Ionic Liquid: Insights Disclosed by Bond Energetic Study Zhen Wang,a,c Fei-Xiang Gao,a Pengju Ji*,a and Jin-Pei Cheng*,a,b aCenter of Basic Molecular Science (CBMS), Department of Chemistry, Tsinghua University, Beijing 100083, China bState Key Laboratory on Elemento-organic Chemistry, Department of Chemistry, Nankai University, Tianjin 300071, China cSchool of Chemical and Environmental Engineering, Anyang Institute of Technology, Anyang 455000, China Email: [email protected]; [email protected] Table of Contents 1. Experimental...............................................................................................................................S2 1.1 Materials and instrumentations.................................................................................................S2 1.2 Synthesis of [DBUH][OTf].......................................................................................................S2 1.3 Synthesis of protic amine salts..................................................................................................S5 1.4 Determination of pKa in [DBUH][OTf] ...................................................................................S7 2. Acidities of benzoic acids in [DBUH][OTf]...............................................................................S13 3. Regression analyses....................................................................................................................S14 -

Glossary of Terms Used in Physical Organic Chemistry (IUPAC Recommendations 1994)
Article Glossary of terms used in physical organic chemistry (IUPAC Recommendations 1994) MULLER, Paul Abstract This glossary contains definitions and explanatory notes for terms used in the context of research and publications in physical organic chemistry. Its aim is to provide guidance on physical organic chemical terminology with a view to achieve a far-reaching consensus on the definitions of useful terms and on the abandonment of unsatisfactory ones. As a consequence of the development of physical organic chemistry, and of the increasing use of physical organic terminology in other fields of chemistry, the 1994 revision of the Glossary is much expanded in comparison to the previous edition, and it also includes terms from cognate fields. A few definitions have been refined, some others totally revised in the light of comments received from the scientific community. Reference MULLER, Paul. Glossary of terms used in physical organic chemistry (IUPAC Recommendations 1994). Pure and Applied Chemistry, 1994, vol. 66, no. 5, p. 1077-1184 DOI : 10.1351/pac199466051077 Available at: http://archive-ouverte.unige.ch/unige:151920 Disclaimer: layout of this document may differ from the published version. 1 / 1 Pure &Appl. Chem., Vol. 66, No. 5, pp. 1077-1184,1994. Printed in Great Britain. 0 1994 IUPAC INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY ORGANIC CHEMISTRY DIVISION COMMISSION ON PHYSICAL ORGANIC CHEMISTRY* GLOSSARY OF TERMS USED IN PHYSICAL ORGANIC CHEMISTRY (IUPAC Recommendations 1994) Prepared for publication by P. MULLER DCpartement de Chimie Organique, UniversitC de Genbve, CH-1211 Genbve 4, Suisse *Membership of the Commission during the period (1987-93) the report was drafted was as follows: Chairman: 1987-93 P. -

Deoxyadenosine the Concentration Dependence of IR Spectra (1800-1400 Cur-) Have Been Found Which Reflects Stacking Association of These Compounds
Volume Number 13 Volume 8 Number 13 19801980 Nucleic Acids Research IR study of base stacking interactions A.A.Maevsky and B.I.Sukhorukov Institute of Biological Physics, USSR Academy of Sciences, Pushchino, Moscow Region 142292, USSR Received 12 May 1980 ABSXTACT For D 0 solutions of 1,3-dimethyluracil, cytidine, caffei- ne, nosin and 2'-deoxyadenosine the concentration dependence of IR spectra (1800-1400 cur-) have been found which reflects stacking association of these compounds. A method is proposed to use this data to obtain thermodynamic parameters of associa- tion and the molecular spectra in the monomer and associated forms. The homoassociation constant for 1,3-dimethyluracil was estimated as k=O.65. Stacking is shown to change radically the spectra, inducing a high-frequency shift of cabonyl vibrati- ons and a decrease in the intensity of skeletal stretching vibration bands. This allows one to distinguish between the stacking interactions and hydrogen bonding. A conclusion is made about the considerable contribution of stacking interac- tions into the change of IR spectra of DNA and other polynucle- otides following conformational transitions. INTRODUCTION In aqueous solutions the mucleic bases and their deriva- tives associate formin the interplanar contacts. The so-call- ed stacking interaction takes place /1/. This type of interac- tion is of great interest for pbysico-chemistry of nucleic acids, since the stacking makes a substantial contribution into the stability of the secondary structure of polynucleotid- es /2-5/. A study of this phenomenon is also necessary to understand the chemical reactivity of the plane molecules and to solve a number of problems of prebiological evolution. -

The Use of Heterocomplementary Hydrogen Bonding Motifs for Supramolecular Materials Chemistry
The Use of Heterocomplementary Hydrogen Bonding Motifs for Supramolecular Materials Chemistry Kelly Ann Houton Submitted in accordance with the requirements for the degree of Doctor of Philosophy The University of Leeds School of Chemistry March 2015 Intellectual Property and Publication Statements The candidate confirms that the work submitted is her own, except where work, which has formed part of jointly-authored publications, has been included. The contribution of the candidate and the other authors to this work has been explicitly indicated below. The candidate confirms that appropriate credit has been given within the thesis where reference has been made to the work of others. Chapter 1- Introduction; adapted from a review article ‘Hydrogen-bonded polyurethanes’, Houton, K.A and Wilson, A. J. Polymer International, 2015; 64, 165-173. The contributions of the authors are as follows: KAH (the candidate) wrote the corresponding sections of the review and produced related figures, whilst AJW edited the manuscript into its present form. Chapter 2- Light-triggered supramolecular self-sorting cascades; the work reported in this chapter formed part of a research paper ‘Sequential and Photo triggered Supramolecular Self-Sorting Cascades Using Hydrogen-Bonded Motifs’, Chemical Science, 2013, 4, 1825-1829. The contributions of the authors are as follows: AJW, MLP and KAH (the candidate) designed the research, KAH (the candidate) performed the research and characterisation of AIC motifs appended with a light- cleavable motif, and drafted the corresponding section of the manuscript. MLP performed the non-triggered self-sorting experiments and AJW edited the manuscript into its present form. Chapter 3- Mechanical properties of supramolecular polymers; the work reported in this chapter formed part of a research paper ‘Tuneable Self-Assembled Elastomers Using Triply Hydrogen-Bonded Arrays’, Macromolecules, 2012, 45, 4723-4729.