Nephritis Responses in Murine and Human Lupus Analysis Defines
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cytogenomic SNP Microarray - Fetal ARUP Test Code 2002366 Maternal Contamination Study Fetal Spec Fetal Cells
Patient Report |FINAL Client: Example Client ABC123 Patient: Patient, Example 123 Test Drive Salt Lake City, UT 84108 DOB 2/13/1987 UNITED STATES Gender: Female Patient Identifiers: 01234567890ABCD, 012345 Physician: Doctor, Example Visit Number (FIN): 01234567890ABCD Collection Date: 00/00/0000 00:00 Cytogenomic SNP Microarray - Fetal ARUP test code 2002366 Maternal Contamination Study Fetal Spec Fetal Cells Single fetal genotype present; no maternal cells present. Fetal and maternal samples were tested using STR markers to rule out maternal cell contamination. This result has been reviewed and approved by Maternal Specimen Yes Cytogenomic SNP Microarray - Fetal Abnormal * (Ref Interval: Normal) Test Performed: Cytogenomic SNP Microarray- Fetal (ARRAY FE) Specimen Type: Direct (uncultured) villi Indication for Testing: Patient with 46,XX,t(4;13)(p16.3;q12) (Quest: EN935475D) ----------------------------------------------------------------- ----- RESULT SUMMARY Abnormal Microarray Result (Male) Unbalanced Translocation Involving Chromosomes 4 and 13 Classification: Pathogenic 4p Terminal Deletion (Wolf-Hirschhorn syndrome) Copy number change: 4p16.3p16.2 loss Size: 5.1 Mb 13q Proximal Region Deletion Copy number change: 13q11q12.12 loss Size: 6.1 Mb ----------------------------------------------------------------- ----- RESULT DESCRIPTION This analysis showed a terminal deletion (1 copy present) involving chromosome 4 within 4p16.3p16.2 and a proximal interstitial deletion (1 copy present) involving chromosome 13 within 13q11q12.12. This -

Network Medicine Approach for Analysis of Alzheimer's Disease Gene Expression Data
International Journal of Molecular Sciences Article Network Medicine Approach for Analysis of Alzheimer’s Disease Gene Expression Data David Cohen y, Alexander Pilozzi y and Xudong Huang * Neurochemistry Laboratory, Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Charlestown, MA 02129, USA; [email protected] (D.C.); [email protected] (A.P.) * Correspondence: [email protected]; Tel./Fax: +1-617-724-9778 These authors contributed equally to this work. y Received: 15 November 2019; Accepted: 30 December 2019; Published: 3 January 2020 Abstract: Alzheimer’s disease (AD) is the most widespread diagnosed cause of dementia in the elderly. It is a progressive neurodegenerative disease that causes memory loss as well as other detrimental symptoms that are ultimately fatal. Due to the urgent nature of this disease, and the current lack of success in treatment and prevention, it is vital that different methods and approaches are applied to its study in order to better understand its underlying mechanisms. To this end, we have conducted network-based gene co-expression analysis on data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database. By processing and filtering gene expression data taken from the blood samples of subjects with varying disease states and constructing networks based on that data to evaluate gene relationships, we have been able to learn about gene expression correlated with the disease, and we have identified several areas of potential research interest. Keywords: Alzheimer’s disease; network medicine; gene expression; neurodegeneration; neuroinflammation 1. Introduction Alzheimer’s disease (AD) is the most widespread diagnosed cause of dementia in the elderly [1]. -

Expression Level and Prognostic Potential of MAP3K8 in Human Cancers Based on Data Mining
Expression Level and Prognostic Potential of MAP3K8 in Human Cancers Based on Data Mining Jiatao Hao Xi'an Jiaotong University Medical College First Aliated Hospital Yumeng Cao China Medical University Hui Yu Xi'an Jiaotong University Medical College First Aliated Hospital Lu Zong Xi'an Jiaotong University Medical College First Aliated Hospital Ruifang An Xi'an Jiaotong University Medical College First Aliated Hospital Yan Xue ( [email protected] ) Xi'an Jiaotong University Medical College First Aliated Hospital https://orcid.org/0000-0002-6061- 1706 Primary research Keywords: MAPK kinase kinase 8 (MAP3K8), expression, methylation, prognosis, protein-protein interaction (PPI), functional enrichment analysis Posted Date: March 9th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-276370/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/22 Abstract Background: MAPK kinase kinase 8 (MAP3K8) is a member of the MAP3K family with a major role in the regulation of the MAPK pathway and immune response. Differential expression of MAP3K8 is closely correlated with tumorigenesis. In this study, we used bioinformatics tools to explore expression level, prognostic values, and interactive networks of MAP3K8 in human cancers. Methods: Expression prole of MAP3K8 was analyzed using the Oncomine Platform, the Gene Expression Proling Interactive Analysis (GEPIA), and UALCAN. Survival analysis was evaluated via UALCAN, GEPIA, and DriverDBv3 databases. Then, MAP3K8 related functional networks were explored within GeneMANIA and Cytoscape. Moreover, Metascape was used to analyze Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways. Results: We found that MAP3K8 was down-expressed in most cancer samples compared with paired normal tissues. -

PIGT Antibody Cat
PIGT Antibody Cat. No.: 26-193 PIGT Antibody Antibody used in WB on Hum. Adult Placenta at 1 ug/ml. Specifications HOST SPECIES: Rabbit SPECIES REACTIVITY: Human, Mouse, Rat Antibody produced in rabbits immunized with a synthetic peptide corresponding a region IMMUNOGEN: of human PIGT. TESTED APPLICATIONS: ELISA, WB PIGT antibody can be used for detection of PIGT by ELISA at 1:62500. PIGT antibody can APPLICATIONS: be used for detection of PIGT by western blot at 1 μg/mL, and HRP conjugated secondary antibody should be diluted 1:50,000 - 100,000. POSITIVE CONTROL: 1) Tranfected 293T Cell Lysate PREDICTED MOLECULAR 64 kDa WEIGHT: October 2, 2021 1 https://www.prosci-inc.com/pigt-antibody-26-193.html Properties PURIFICATION: Antibody is purified by peptide affinity chromatography method. CLONALITY: Polyclonal CONJUGATE: Unconjugated PHYSICAL STATE: Liquid Purified antibody supplied in 1x PBS buffer with 0.09% (w/v) sodium azide and 2% BUFFER: sucrose. CONCENTRATION: batch dependent For short periods of storage (days) store at 4˚C. For longer periods of storage, store PIGT STORAGE CONDITIONS: antibody at -20˚C. As with any antibody avoid repeat freeze-thaw cycles. Additional Info OFFICIAL SYMBOL: PIGT ALTERNATE NAMES: PIGT, CGI-06, FLJ41596, MGC8909, NDAP, PNH2, MCAHS3 ACCESSION NO.: NP_057021 PROTEIN GI NO.: 23397653 GENE ID: 51604 USER NOTE: Optimal dilutions for each application to be determined by the researcher. Background and References PIGT is a protein that is involved in glycosylphosphatidylinositol (GPI)-anchor biosynthesis. The GPI-anchor is a glycolipid found on many blood cells and serves to anchor proteins to the cell surface. -

Genome-Wide Analysis of Differential Transcriptional and Epigenetic
bioRxiv preprint doi: https://doi.org/10.1101/083246; this version posted October 26, 2016. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC 4.0 International license. 1 Genome-wide Analysis of Differential Transcriptional and 2 Epigenetic Variability Across Human Immune Cell Types 3 Simone Ecker,1,2,* Lu Chen,3,4 Vera Pancaldi,1 Frederik O. Bagger,4,5,6 José María Fernández,1 Enrique Carrillo de 4 Santa Pau,1 David Juan,1 Alice L. Mann,3 Stephen Watt,3 Francesco Paolo Casale,6 Nikos Sidiropoulos,7,8,9 Nicolas 5 Rapin,7,8,9 Angelika Merkel,10 BLUEPRINT Consortium, Henk Stunnenberg,11 Oliver Stegle,6 Mattia Frontini,4,5,12 Kate 6 Downes,4,5 Tomi Pastinen,13 Taco W. Kuijpers,14,15 Daniel Rico,1,17 Alfonso Valencia,1,17 Stephan Beck,2,17 Nicole 7 Soranzo3,4,17,* and Dirk S. Paul2,16,17,* 8 9 1Structural Biology and Biocomputing Programme, Spanish National Cancer Research Center (CNIO), Melchor Fernández Almagro 3, 28029 Madrid, Spain 10 2UCL Cancer Institute, University College London, 72 Huntley Street, London, WC1E 6BT, UK 11 3Department of Human Genetics, Wellcome Trust Sanger Institute, Wellcome Trust Genome Campus, Hinxton, Cambridge, CB10 1HH, UK 12 4Department of Haematology, University of Cambridge, Cambridge Biomedical Campus, Long Road, Cambridge, CB2 0PT, UK 13 5National Health Service (NHS) Blood and Transplant, Cambridge Biomedical Campus, Long Road, Cambridge, CB2 -

KLF6 Depletion Promotes NF-Κb Signaling in Glioblastoma
OPEN Oncogene (2017) 36, 3562–3575 www.nature.com/onc ORIGINAL ARTICLE KLF6 depletion promotes NF-κB signaling in glioblastoma AP Masilamani1,2, R Ferrarese1,2, E Kling1,2, NK Thudi3, H Kim4, DM Scholtens5, F Dai1,2, M Hadler1,2, T Unterkircher1,2, L Platania1,2, A Weyerbrock1,2, M Prinz6,7, GY Gillespie8, GR Harsh IV9, M Bredel3,10 and MS Carro1,2,10 Dysregulation of the NF-κB transcription factor occurs in many cancer types. Krüppel-like family of transcription factors (KLFs) regulate the expression of genes involved in cell proliferation, differentiation and survival. Here, we report a new mechanism of NF- κB activation in glioblastoma through depletion of the KLF6 tumor suppressor. We show that KLF6 transactivates multiple genes negatively controlling the NF-κB pathway and consequently reduces NF-κB nuclear localization and downregulates NF-κB targets. Reconstitution of KLF6 attenuates their malignant phenotype and induces neural-like differentiation and senescence, consistent with NF-κB pathway inhibition. KLF6 is heterozygously deleted in 74.5% of the analyzed glioblastomas and predicts unfavorable patient prognosis suggesting that haploinsufficiency is a clinically relevant means of evading KLF6-dependent regulation of NF-κB. Together, our study identifies a new mechanism by which KLF6 regulates NF-κB signaling, and how this mechanism is circumvented in glioblastoma through KLF6 loss. Oncogene (2017) 36, 3562–3575; doi:10.1038/onc.2016.507; published online 6 February 2017 INTRODUCTION coding region have been controversial.16,18–22 KLF6 has been The NF-κB transcription factor family is oncogenic through proposed to perform its tumor suppression function by promoting suppression of programmed cell death, and promotion of tumor G1 cell cycle arrest mainly through cyclin-dependent kinase 15 growth and invasion.1 In tumors, NF-κB can be activated by inhibitor 1A (CDKN1A) promoter transactivation. -
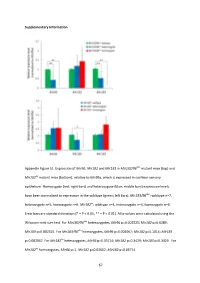
And Mir183 in Mir183/96 Dko Mutant Mice (Top) And
Supplementary Information Appendix Figure S1. Expression of Mir96 , Mir182 and Mir183 in Mir183/96 dko mutant mice (top) and Mir182 ko mutant mice (bottom), relative to Mir99a , which is expressed in cochlear sensory epithelium. Homozygote (red; right bars) and heterozygote (blue; middle bars) expression levels have been normalised to expression in the wildtype (green; left bars). Mir183/96 dko : wildtype n=7, heterozygote n=5, homozygote n=6. Mir182 ko : wildtype n=4, heterozygote n=4, homozygote n=4. Error bars are standard deviation (* = P < 0.05, ** = P < 0.01). All p-values were calculated using the Wilcoxon rank sum test. For Mir183/96 dko heterozygotes, Mir96 p=0.002525; Mir182 p=0.6389; Mir183 p=0.002525. For Mir183/96 dko homozygotes, Mir96 p=0.002067; Mir182 p=0.1014; Mir183 p=0.002067. For Mir182 ko heterozygotes, Mir96 p=0.05714; Mir182 p=0.3429; Mir183 p=0.3429. For Mir182 ko homozygotes, Mir96 p=1; Mir182 p=0.02652; Mir183 p=0.05714. 67 68 Appendix Figure S2. Individual ABR thresholds of wildtype, heterozygous and homozygous Mir183/96 dko mice at all ages tested. Number of mice of each genotype tested at each age is shown on the threshold plot. 69 70 Appendix Figure S3. Individual ABR thresholds of wildtype, heterozygous and homozygous Mir182 ko mice at all ages tested. Number of mice of each genotype tested at each age is shown on the threshold plot. 71 Appendix Figure S4. Mean ABR waveforms at 12kHz, shown at 20dB (top) and 50dB (bottom) above threshold (sensation level, SL) ± standard deviation, at four weeks old. -

NICU Gene List Generator.Xlsx
Neonatal Crisis Sequencing Panel Gene List Genes: A2ML1 - B3GLCT A2ML1 ADAMTS9 ALG1 ARHGEF15 AAAS ADAMTSL2 ALG11 ARHGEF9 AARS1 ADAR ALG12 ARID1A AARS2 ADARB1 ALG13 ARID1B ABAT ADCY6 ALG14 ARID2 ABCA12 ADD3 ALG2 ARL13B ABCA3 ADGRG1 ALG3 ARL6 ABCA4 ADGRV1 ALG6 ARMC9 ABCB11 ADK ALG8 ARPC1B ABCB4 ADNP ALG9 ARSA ABCC6 ADPRS ALK ARSL ABCC8 ADSL ALMS1 ARX ABCC9 AEBP1 ALOX12B ASAH1 ABCD1 AFF3 ALOXE3 ASCC1 ABCD3 AFF4 ALPK3 ASH1L ABCD4 AFG3L2 ALPL ASL ABHD5 AGA ALS2 ASNS ACAD8 AGK ALX3 ASPA ACAD9 AGL ALX4 ASPM ACADM AGPS AMELX ASS1 ACADS AGRN AMER1 ASXL1 ACADSB AGT AMH ASXL3 ACADVL AGTPBP1 AMHR2 ATAD1 ACAN AGTR1 AMN ATL1 ACAT1 AGXT AMPD2 ATM ACE AHCY AMT ATP1A1 ACO2 AHDC1 ANK1 ATP1A2 ACOX1 AHI1 ANK2 ATP1A3 ACP5 AIFM1 ANKH ATP2A1 ACSF3 AIMP1 ANKLE2 ATP5F1A ACTA1 AIMP2 ANKRD11 ATP5F1D ACTA2 AIRE ANKRD26 ATP5F1E ACTB AKAP9 ANTXR2 ATP6V0A2 ACTC1 AKR1D1 AP1S2 ATP6V1B1 ACTG1 AKT2 AP2S1 ATP7A ACTG2 AKT3 AP3B1 ATP8A2 ACTL6B ALAS2 AP3B2 ATP8B1 ACTN1 ALB AP4B1 ATPAF2 ACTN2 ALDH18A1 AP4M1 ATR ACTN4 ALDH1A3 AP4S1 ATRX ACVR1 ALDH3A2 APC AUH ACVRL1 ALDH4A1 APTX AVPR2 ACY1 ALDH5A1 AR B3GALNT2 ADA ALDH6A1 ARFGEF2 B3GALT6 ADAMTS13 ALDH7A1 ARG1 B3GAT3 ADAMTS2 ALDOB ARHGAP31 B3GLCT Updated: 03/15/2021; v.3.6 1 Neonatal Crisis Sequencing Panel Gene List Genes: B4GALT1 - COL11A2 B4GALT1 C1QBP CD3G CHKB B4GALT7 C3 CD40LG CHMP1A B4GAT1 CA2 CD59 CHRNA1 B9D1 CA5A CD70 CHRNB1 B9D2 CACNA1A CD96 CHRND BAAT CACNA1C CDAN1 CHRNE BBIP1 CACNA1D CDC42 CHRNG BBS1 CACNA1E CDH1 CHST14 BBS10 CACNA1F CDH2 CHST3 BBS12 CACNA1G CDK10 CHUK BBS2 CACNA2D2 CDK13 CILK1 BBS4 CACNB2 CDK5RAP2 -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -

The Genetic Landscape of the Human Solute Carrier (SLC) Transporter Superfamily
Human Genetics (2019) 138:1359–1377 https://doi.org/10.1007/s00439-019-02081-x ORIGINAL INVESTIGATION The genetic landscape of the human solute carrier (SLC) transporter superfamily Lena Schaller1 · Volker M. Lauschke1 Received: 4 August 2019 / Accepted: 26 October 2019 / Published online: 2 November 2019 © The Author(s) 2019 Abstract The human solute carrier (SLC) superfamily of transporters is comprised of over 400 membrane-bound proteins, and plays essential roles in a multitude of physiological and pharmacological processes. In addition, perturbation of SLC transporter function underlies numerous human diseases, which renders SLC transporters attractive drug targets. Common genetic polymorphisms in SLC genes have been associated with inter-individual diferences in drug efcacy and toxicity. However, despite their tremendous clinical relevance, epidemiological data of these variants are mostly derived from heterogeneous cohorts of small sample size and the genetic SLC landscape beyond these common variants has not been comprehensively assessed. In this study, we analyzed Next-Generation Sequencing data from 141,456 individuals from seven major human populations to evaluate genetic variability, its functional consequences, and ethnogeographic patterns across the entire SLC superfamily of transporters. Importantly, of the 204,287 exonic single-nucleotide variants (SNVs) which we identifed, 99.8% were present in less than 1% of analyzed alleles. Comprehensive computational analyses using 13 partially orthogonal algorithms that predict the functional impact of genetic variations based on sequence information, evolutionary conserva- tion, structural considerations, and functional genomics data revealed that each individual genome harbors 29.7 variants with putative functional efects, of which rare variants account for 18%. Inter-ethnic variability was found to be extensive, and 83% of deleterious SLC variants were only identifed in a single population. -

Plasma Fatty Acid Ratios Affect Blood Gene Expression Profiles - a Cross-Sectional Study of the Norwegian Women and Cancer Post-Genome Cohort
Plasma Fatty Acid Ratios Affect Blood Gene Expression Profiles - A Cross-Sectional Study of the Norwegian Women and Cancer Post-Genome Cohort Karina Standahl Olsen1*, Christopher Fenton2, Livar Frøyland3, Marit Waaseth4, Ruth H. Paulssen2, Eiliv Lund1 1 Department of Community Medicine, University of Tromsø, Tromsø, Norway, 2 Department of Clinical Medicine, University of Tromsø, Tromsø, Norway, 3 National Institute of Nutrition and Seafood Research (NIFES), Bergen, Norway, 4 Department of Pharmacy, University of Tromsø, Tromsø, Norway Abstract High blood concentrations of n-6 fatty acids (FAs) relative to n-3 FAs may lead to a ‘‘physiological switch’’ towards permanent low-grade inflammation, potentially influencing the onset of cardiovascular and inflammatory diseases, as well as cancer. To explore the potential effects of FA ratios prior to disease onset, we measured blood gene expression profiles and plasma FA ratios (linoleic acid/alpha-linolenic acid, LA/ALA; arachidonic acid/eicosapentaenoic acid, AA/EPA; and total n-6/n-3) in a cross-section of middle-aged Norwegian women (n = 227). After arranging samples from the highest values to the lowest for all three FA ratios (LA/ALA, AA/EPA and total n-6/n-3), the highest and lowest deciles of samples were compared. Differences in gene expression profiles were assessed by single-gene and pathway-level analyses. The LA/ALA ratio had the largest impact on gene expression profiles, with 135 differentially expressed genes, followed by the total n-6/ n-3 ratio (125 genes) and the AA/EPA ratio (72 genes). All FA ratios were associated with genes related to immune processes, with a tendency for increased pro-inflammatory signaling in the highest FA ratio deciles. -

The Genetics of Bipolar Disorder
Molecular Psychiatry (2008) 13, 742–771 & 2008 Nature Publishing Group All rights reserved 1359-4184/08 $30.00 www.nature.com/mp FEATURE REVIEW The genetics of bipolar disorder: genome ‘hot regions,’ genes, new potential candidates and future directions A Serretti and L Mandelli Institute of Psychiatry, University of Bologna, Bologna, Italy Bipolar disorder (BP) is a complex disorder caused by a number of liability genes interacting with the environment. In recent years, a large number of linkage and association studies have been conducted producing an extremely large number of findings often not replicated or partially replicated. Further, results from linkage and association studies are not always easily comparable. Unfortunately, at present a comprehensive coverage of available evidence is still lacking. In the present paper, we summarized results obtained from both linkage and association studies in BP. Further, we indicated new potential interesting genes, located in genome ‘hot regions’ for BP and being expressed in the brain. We reviewed published studies on the subject till December 2007. We precisely localized regions where positive linkage has been found, by the NCBI Map viewer (http://www.ncbi.nlm.nih.gov/mapview/); further, we identified genes located in interesting areas and expressed in the brain, by the Entrez gene, Unigene databases (http://www.ncbi.nlm.nih.gov/entrez/) and Human Protein Reference Database (http://www.hprd.org); these genes could be of interest in future investigations. The review of association studies gave interesting results, as a number of genes seem to be definitively involved in BP, such as SLC6A4, TPH2, DRD4, SLC6A3, DAOA, DTNBP1, NRG1, DISC1 and BDNF.