(12) Patent Application Publication (10) Pub. No.: US 2012/0164068 A1 Hudson Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Personal Music Collection
Christopher Lee :: Personal Music Collection electricshockmusic.com :: Saturday, 25 September 2021 < Back Forward > Christopher Lee's Personal Music Collection | # | A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | | DVD Audio | DVD Video | COMPACT DISCS Artist Title Year Label Notes # Digitally 10CC 10cc 1973, 2007 ZT's/Cherry Red Remastered UK import 4-CD Boxed Set 10CC Before During After: The Story Of 10cc 2017 UMC Netherlands import 10CC I'm Not In Love: The Essential 10cc 2016 Spectrum UK import Digitally 10CC The Original Soundtrack 1975, 1997 Mercury Remastered UK import Digitally Remastered 10CC The Very Best Of 10cc 1997 Mercury Australian import 80's Symphonic 2018 Rhino THE 1975 A Brief Inquiry Into Online Relationships 2018 Dirty Hit/Polydor UK import I Like It When You Sleep, For You Are So Beautiful THE 1975 2016 Dirty Hit/Interscope Yet So Unaware Of It THE 1975 Notes On A Conditional Form 2020 Dirty Hit/Interscope THE 1975 The 1975 2013 Dirty Hit/Polydor UK import {Return to Top} A A-HA 25 2010 Warner Bros./Rhino UK import A-HA Analogue 2005 Polydor Thailand import Deluxe Fanbox Edition A-HA Cast In Steel 2015 We Love Music/Polydor Boxed Set German import A-HA East Of The Sun West Of The Moon 1990 Warner Bros. German import Digitally Remastered A-HA East Of The Sun West Of The Moon 1990, 2015 Warner Bros./Rhino 2-CD/1-DVD Edition UK import 2-CD/1-DVD Ending On A High Note: The Final Concert Live At A-HA 2011 Universal Music Deluxe Edition Oslo Spektrum German import A-HA Foot Of The Mountain 2009 Universal Music German import A-HA Hunting High And Low 1985 Reprise Digitally Remastered A-HA Hunting High And Low 1985, 2010 Warner Bros./Rhino 2-CD Edition UK import Digitally Remastered Hunting High And Low: 30th Anniversary Deluxe A-HA 1985, 2015 Warner Bros./Rhino 4-CD/1-DVD Edition Boxed Set German import A-HA Lifelines 2002 WEA German import Digitally Remastered A-HA Lifelines 2002, 2019 Warner Bros./Rhino 2-CD Edition UK import A-HA Memorial Beach 1993 Warner Bros. -

UNIVERSAL MUSIC • Royal Wood – Ghost Light • Rufus Wainwright
Royal Wood – Ghost Light Rufus Wainwright – Take All My Loves: 9 Shakespeare Sonnets André Rieu – Magic Of The Waltz Check out new releases in our Vinyl Section! New Releases From Classics And Jazz Inside!!! And more… UNI16-15 UNIVERSAL MUSIC 2450 Victoria Park Ave., Suite 1, Willowdale, Ontario M2J 5H3 Phone: (416) 718.4000 *Artwork shown may not be final UNIVERSAL MUSIC CANADA NEW RELEASE Artist/Title: Various Artists / Now! 26 Bar Code: Cat. #: 0254782454 Price Code: G Order Due: March 3, 2016 Release Date: April 1, 2016 6 02547 82454 7 File: Pop Genre Code: 33 Box Lot: 25 Key Tracks: SUPER SHORT SELL Key Points: National Major TV, Radio Online Advertising Campaign Now! Brand is consistently one of the strongest and best‐selling compilations every year The NOW! brand has generated sales of over 200 million albums worldwide Sold over 4 million copies in Canada since its debut. Includes: Justin Bieber – Sorry Selena Gomez ‐ Same Old Love Coleman Hell ‐ 2 Heads Shawn Mendes ‐ Stitches Ellie Goulding ‐ On My Mind Alessia Cara ‐ Here DNCE ‐ Cake By The Ocean Demi Lovato ‐ Confident Nathaniel Rateliff & The Night Sweats ‐ S.O.B Hedley ‐ Hello James Bay ‐ Let It Go Mike Posner ‐ I Took A Pill In Ibiza And more….. Also Available: Artist/Title: Various / Now! 25 Cat#: 0254750866 Price Code: JSP UPC#: 02547 50866 6 9 INTERNAL USE Label Name: Universal Music Canada Territory: Domestic Release Type: O For additional artist information please contact Nick at 416‐718‐4045 or [email protected] UNIVERSAL MUSIC 2450 Victoria Park Avenue, Suite 1, Toronto, ON M2J 5H3 Phone: (416) 718‐4000 Fax: (416) 718‐4218 UNIVERS AL M USI C CA NAD A N EW RELEASE Artist/Title: THE STRUMBELLAS / HOPE (CD) Cat. -
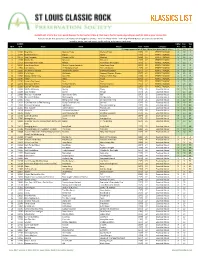
KLASSICS LIST Criteria
KLASSICS LIST criteria: 8 or more points (two per fan list, two for U-Man A-Z list, two to five for Top 95, depending on quartile); 1984 or prior release date Sources: ten fan lists (online and otherwise; see last page for details) + 2011-12 U-Man A-Z list + 2014 Top 95 KSHE Klassics (as voted on by listeners) sorted by points, Fan Lists count, Top 95 ranking, artist name, track name SLCRPS UMan Fan Top ID # ID # Track Artist Album Year Points Category A-Z Lists 95 35 songs appeared on all lists, these have green count info >> X 10 n 1 12404 Blue Mist Mama's Pride Mama's Pride 1975 27 PERFECT KLASSIC X 10 1 2 12299 Dead And Gone Gypsy Gypsy 1970 27 PERFECT KLASSIC X 10 2 3 11672 Two Hangmen Mason Proffit Wanted 1969 27 PERFECT KLASSIC X 10 5 4 11578 Movin' On Missouri Missouri 1977 27 PERFECT KLASSIC X 10 6 5 11717 Remember the Future Nektar Remember the Future 1973 27 PERFECT KLASSIC X 10 7 6 10024 Lake Shore Drive Aliotta Haynes Jeremiah Lake Shore Drive 1971 27 PERFECT KLASSIC X 10 9 7 11654 Last Illusion J.F. Murphy & Salt The Last Illusion 1973 27 PERFECT KLASSIC X 10 12 8 13195 The Martian Boogie Brownsville Station Brownsville Station 1977 27 PERFECT KLASSIC X 10 13 9 13202 Fly At Night Chilliwack Dreams, Dreams, Dreams 1977 27 PERFECT KLASSIC X 10 14 10 11696 Mama Let Him Play Doucette Mama Let Him Play 1978 27 PERFECT KLASSIC X 10 15 11 11547 Tower Angel Angel 1975 27 PERFECT KLASSIC X 10 19 12 11730 From A Dry Camel Dust Dust 1971 27 PERFECT KLASSIC X 10 20 13 12131 Rosewood Bitters Michael Stanley Michael Stanley 1972 27 PERFECT -

Gamma Gamma 2 Mp3, Flac, Wma
Gamma Gamma 2 mp3, flac, wma DOWNLOAD LINKS (Clickable) Genre: Rock Album: Gamma 2 Country: Russia Released: 2002 Style: Hard Rock MP3 version RAR size: 1603 mb FLAC version RAR size: 1628 mb WMA version RAR size: 1760 mb Rating: 4.2 Votes: 639 Other Formats: MP2 WMA MP4 DXD TTA XM AC3 Tracklist Hide Credits 1 Mean Streak 4:51 2 Four Horsemen 4:48 Dirty City 3 4:05 Vocals [Additional] – Genya Ravan 4 Voyager 5:24 Something In The Air 5 3:17 Written-By – John Keen* 6 Cat On A Leash 4:04 7 Skin And Bone 4:49 8 Mayday 5:38 Credits Bass Guitar – Glenn Letsch Design [Package], Art Direction – Mick Haggerty Drums – Denny Carmassi Engineer – Gary Lyons Engineer [Assistant] – Ken Kessie, Peter Thea*, Wayne Lewis Guitar – Ronnie Montrose Mastered By – George Marino Photography By [Cover, Inner Sleeve] – Jeffrey Scales Photography By [Cover] – Mick Haggerty Producer – Gary Lyons, Ronnie Montrose Synthesizer – Jim Alcivar Vocals – Davey Pattison Written-By – D. Pattison* (tracks: 1 to 4), J. Stahl* (tracks: 6, 7), J. Alcivar* (tracks: 1, 8), R. Montrose* (tracks: 1 to 4, 6 to 8) Notes High-quality factory made CD, 4-pages booklet, standart jewel case. Barcode and Other Identifiers Barcode: 664140628823 Matrix / Runout: Z12108 45 WOU2 6288.2-2 01 Other versions Category Artist Title (Format) Label Category Country Year 6E-288 Gamma Gamma 2 (LP, Album) Elektra 6E-288 US 1980 Gamma 2 (LP, Album, 6E-288 Gamma Elektra 6E-288 US 1980 Promo) ELK 52 245 Gamma Gamma 2 (LP, Album) Elektra ELK 52 245 Germany 1980 Gamma 2 (CD, Album, 2000 FruitGum FCCD 109121-2 Gamma FCCD 109121-2 Russia 2002 Unofficial) Corp. -

Rescue 4A Miracle' Iraqis Seize Port, Bomb Tehran Sweitzer, Wilson
Rescue 4a miracle' VALDEZ, Alaska (UPI) - With all 519 foot waves and they just go right up and right rescued passengers and crew members safely down." ashore from the crippled luxury liner Most of the passengers and crew, all put Prinsendam, the Coast Guard set out Monday ashore at the towns of Sitka or Valdez, awaited to save the ship, burning and adrift in the Gulf flights to Seattle to get them started on their of Alaska. *'»y home.The Coast Guard revised the total The cutter Mellon went along side the listing of passengers and crew from 506 to 519. vessel at daybreak and put aboard a Coast Dense fog at the oil port of Valdez, where Guard team to assess damage. A firefighting 227 rescued passengers waited, prevented crew from the cutter was ready to board and planes from arriving to begin a shuttle service begin dousing flames to save the $25 million to Anchorage and on to Seattle. vessel, a spokesman said. Passengers and officials marveled at the success of the rescue, termed the biggest of a Related story, p. 5 single ship in modern maritime history. Coast Guard helicopter pilot Lt. Bruce Two Coast Guard helicopters also headed for Melnick. Who pulled 109 people from lifeboats, the ship, drifting 145 miles west off Cape called it "a miracle everybody was all right." Spencer on the southeastern Alaska coast. The last persons to be rescued, a lifeboat Weather was reported subdued compared to C-J***--"*& contingent of 20 persons, spent about 21 hours the high winds and 25-foot seas rescuers bobbing on the rough seas before they were contended with when pulling survivors from rescued. -

3. No Mercy the Day That You Abandoned Me My Whole World Fell Apart but As Time Goes by I Came to See That You Were Cold at Heart
3. No mercy The day that you abandoned me my whole world fell apart But as time goes by I came to see that you were cold at heart I thought I gave you everything At least all I could supply I thought I was a bit more than A stranger passing by Words I cannot speak Sounds I cannot hear Views I cannot see (No mercy) Hopes I cannot hope Dreams I cannot dream Senses I can’t feel (No mercy) Thoughts I cannot think Tears I cannot cry Steps I cannot take (No mercy) 1. Mother Nature's accident When I come to find When I first met you, you were telling me about your plans What you left behind 'Bout leaving your country, leaving family and friends One thing on my mind: No mercy! But you were just bluffing, they didn't need you there You had no education and they didn't like your hair As your image fades I realize I did not clearly see I thought I could rely on you but you took advantage of me Chorus: So if you think you are my friend Words I cannot speak You're mother Nature's accident Sounds I cannot hear Views I cannot see (No mercy) Always talkin' 'bout yourself, not listening to another Gifts I cannot bring You don't hardly know the difference from a whore and your lover Songs I cannot sing Never trusting anyone, always arguing about things Thoughts I cannot cling to (No mercy) Wasting all your money on gambling and drinks Making fun of all your partners, being rude to those you meet, uh! My hands were tied, could not escape from you You just don’t want to realize that it's them you need The dope was gone, just like you asked me to We came