The Structure of Catalyst Gauzes After Hydrogen Cyanide Production
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Jaime Gómez Díaz
FIRST-PRINCIPLES MECHANISTIC STUDIES OF AMMONIA-RELATED INDUSTRIAL PROCESSES Jaime Gómez Díaz ISBN: 978-84-694-1247-3 Dipòsit Legal: T-317-2011 ADVERTIMENT. La consulta d’aquesta tesi queda condicionada a l’acceptació de les següents condicions d'ús: La difusió d’aquesta tesi per mitjà del servei TDX (www.tesisenxarxa.net) ha estat autoritzada pels titulars dels drets de propietat intel·lectual únicament per a usos privats emmarcats en activitats d’investigació i docència. No s’autoritza la seva reproducció amb finalitats de lucre ni la seva difusió i posada a disposició des d’un lloc aliè al servei TDX. No s’autoritza la presentació del seu contingut en una finestra o marc aliè a TDX (framing). Aquesta reserva de drets afecta tant al resum de presentació de la tesi com als seus continguts. En la utilització o cita de parts de la tesi és obligat indicar el nom de la persona autora. ADVERTENCIA. La consulta de esta tesis queda condicionada a la aceptación de las siguientes condiciones de uso: La difusión de esta tesis por medio del servicio TDR (www.tesisenred.net) ha sido autorizada por los titulares de los derechos de propiedad intelectual únicamente para usos privados enmarcados en actividades de investigación y docencia. No se autoriza su reproducción con finalidades de lucro ni su difusión y puesta a disposición desde un sitio ajeno al servicio TDR. No se autoriza la presentación de su contenido en una ventana o marco ajeno a TDR (framing). Esta reserva de derechos afecta tanto al resumen de presentación de la tesis como a sus contenidos. -
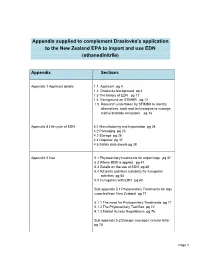
Appendix Supplied to Complement Draslovka's Application to the New
Appendix supplied to complement Draslovka’s application to the New Zealand EPA to import and use EDN (ethanedinitrile) Appendix Sections Appendix 1 Applicant details 1.1. Applicant pg 4 1.2 Draslovka background pg 4 1.3 The history of EDN pg 11 1.4 Background on STIMBR pg 12 1.5. Research undertaken by STIMBR to identify alternatives, tools and technologies to manage methyl bromide emissions pg 16 Appendix 4 Life cycle of EDN 4.1 Manufacturing and Importation pg 24 4.2 Packaging pg 25 4.3 Storage pg 28 4.4 Disposal pg 37 4.5 Safety data sheets pg 38 Appendix 5 Use 5.1 Phytosanitary treatments for export logs pg 47 5.2 Where EDN is applied pg 47 5.3 Details on the use of EDN pg 48 5.4 NZ ports and their suitability for fumigation activities pg 50 5.5 Fumigation with EDN pg 60 Sub appendix 5.1 Phytosanitary Treatments for logs exported from New Zealand pg 71 5.1.1 The need for Phytosanitary Treatments pg 71 5.1.2 The Phytosanitary Tool Box pg 72 5.1.3 Market Access Negotiations pg 76 Sub appendix 5.2 Draeger cyanogen circular letter pg 78 Page 1 Appendix 6 Toxicology and HSNO 6.1 Classification pg 79 hazard classifications of the formulated 6.2 Toxicology pg 87 substance 6.2.1 Mode of action and toxic kinetic pg 87 6.2.2 Acute toxicity pg 88 Acute inhalation Oral toxicity Dermal toxicity Eye irritancy 6.2.3 Systemic toxicity pg 90 6.2.4 Reproductive and developmental toxicity pg 90 6.2.5 Neurotoxicity pg 91 6.2.6 Chronic toxicity pg 92 6.2.7 Genotoxicity pg 93 6.2.8 Carcinogenicity pg 95 6.2.9 WES and TEL values pg 96 6.3 Eco-toxicology 6.3.1 Fate in the environment pg 111 6.3.2 Ecotoxicological Classifications pg 113 Appendix 7 7.2 Risks costs and benefits analysis of 7.2.1 Risks pg 114 Introduction Proposed use Product description: and approach to risk analysis Physical characteristic assessment Human toxicological assessment Ecotoxicology risk assessment 7.2.2 Costs pg 137 Associated with a new chemical. -
Cyanide Process
Cyanide Process The cyanide antidote kit consists of three medications given together: amyl nitrite, sodium nitrite, and sodium thiosulfate. Sodium cyanide solution in water is a strong base; it reacts violently with acid and is corrosive. How is cyanide waste treated at your facility? Chemically speaking it looks like this CN + 2H20 -> NH3 + HCOO in laymen's terms we destroy cyanide using Thermal Hydrolysis. Liquid – vapor diagram of the system H 2O– HCN at atmospheric. The process was invented in 1887 by the Scottish chemists John S. 1 word related to cyanide process: industrial process. net dictionary. txt) or read online for free. Separate the process into half reactions.. 7k points). Australia is a major global manufacturer and exporter of sodium cyanide, with manufacturing facilities in western Australia. Orica's world leading cyanide analysers have been designed to optimise cyanide usage and lower costs, whilst maintaining precious metal recovery rates. Cyanide in fruits and vegetables is in the form of cyanogenic glycosides (cyanoglycosides). A cyanide is a compound, known simply as a 'cyanide', that contains a cyanide group (CN-) 1,3-6. After the door is sealed, and when the warden gives the signal, the executioner in a separate room operates a lever that releases the cyanide into the liquid. Whilst hanging is one of the most reliable methods of suicide, as for firearms, it is not 100% effective - studies would suggest 77% - 88% effective 1. 1 word related to cyanide process: industrial process. For the gold concentrate cyanide plant, the CaO concentration of the general control leaching process is between 2 and 5/10,000.