INHALATIONAL ANAESTHETIC AGENTS (A) to Describe the Principles of Vaporisation of Inhalational Agents
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
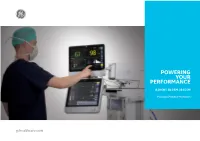
Powering Your Performance
POWERING YOUR PERFORMANCE B105M | B125M | B155M Modular Patient Monitors gehealthcare.com Advanced capabilities. Simple scalability. Dependable technology. B1x5M range of modular patient monitors deliver premium clinical performance across care areas. Scalable platform You can monitor essential vital signs (ECG, SpO2, Temp, NIBP, RR) and easily scale to advanced parameter modules like respiratory gases and Clinical precision anesthetic agents, NMT and Entropy™ – up to three advanced parameters simultaneously. These accurate, reliable, and easy-to-use monitors Intuitive design enable simple and intuitive workflow with a choice of 10-, 12- or 15-inch touch screen displays. B1x5M range of monitors come with Value Dependable technology Software Platform (VSP 3.0). Legacy of premium clinical performance. Excellence of advanced features. Timely clinical decisions. GE Healthcare’s more than 45 years of clinical excellence in The B1x5M range of modular patient monitors helps you quickly designing reliable patient monitoring systems provides you with take charge of patient conditions like arrhythmia and high/low advanced features, such as: blood pressure, and efficiently assess the level of consciousness. They seamlessly integrate with the CARESCAPE Ecosystem for centralized alarm management and efficient workflow. • DINAMAP™ SuperSTAT™ NIBP • EK-Pro v14 algorithm The solutions allow you to effectively monitor deteriorating • CO sidestream and cardiac output patient conditions and make timely interventions by tracking the 2 National Early Warning -

Using Entropy in the General Anesthesia Managements
Research Article Clinics in Surgery Published: 07 Jul, 2017 Using Entropy in the General Anesthesia Managements Elif Büyükerkmen, Remziye Gül Sivaci and Elif Doğan Baki* Departement of Anesthesiology and Reanimation, Afyon Kocatepe University, Afyonkarahisar, Turkey Abstract Purpose: We aimed to investigate the effects of three general anesthesic management on depth of anesthesia, anesthesic quality, agent consumption and postoperative recovery. Materials and Methods: 90 patients scheduled for elective tympanoplasty and septoplasty surgery with American Society of Anesthesiologist (ASA) pysical status between I-III were included in this study. Neuromuscular transmission (NMT), surgical pleth index (SPI) and entropy were monitorized in addition to standart monitoring. Entropy was recorded as state entropy (SE) and response entropy (RE). After standart anesthesia induction, patients were divided into three groups according to maintenance of anesthesia using a sealed envelope system. Propofol 3-5 mg/kg/h iv infusion was performed to Group 1 (Group P, n=30), Desflurane 1MAC was used to Group 2 (Group D, n=30) and Sevoflurane to Group 3 (Group S, n=30). Also, rocuronium and remifentanyl infusion were used in maintenance. While desflurane and sevoflurane consumption were recorded from the anesthesia directly, propofol consumption was calculated through the consumption of perfusors and recorded at the end of the surgery. Total cost of anesthetics that used were calculated by multiplying the unit price with their consumption. Apart from these, hemodynamic values of all patients, recovery time, alertness levels in the recovery room (according to Ramsey Scale) were recorded. Results: Significant differences were found between the three groups in terms of cost. While the cost of propofol was significantly lower, it was significantly higher in desfluane group. -

The Effect of Spectral Entropy Monitoring on Propofol Use and Recovery in Children
Anesth Pain Med 2014; 9: 138-143 ■Clinical Research■ The effect of spectral entropy monitoring on propofol use and recovery in children Department of Anesthesiology and Pain Medicine, Dong-A University College of Medicine, *Myung Sung Pain Clinic, Busan, Korea Ji-yeon Lee, So Ron Choi, Chan Jong Chung, Ji Hyeon Lee, Ji-hye Park, and Chang-Yeoul Baik* Background: The evaluation of anesthetic depth using electro- use. Now, however, anesthesia depth can be measured using encephalography showed reduction in recovery time from anesthe- electroencephalography (EEG)-based devices, such as bispectral sia and decrease in the amount of anesthesia used. This research index (BIS), and spectral entropy; these monitors can indicate compared the dosage of propofol and the recovery characteristics when anesthesia was controlled using spectral entropy monitoring the level of consciousness during anesthesia [1-4]. One study and when it was controlled by hemodynamic changes. demonstrated that the control of anesthesia using BIS monitoring Methods: Seventy children of the American Society of Anesthes- decreased the anesthetic use and shortened recovery time [5]. iologists physical class I–II, ages 3–10, that were scheduled for general anesthesia were randomly distributed into two groups. The Entropy measurements reflect EEG signal irregularity. With children were sedated with midazolam (0.15 mg/kg), and anesthesia increasing anesthesia depth, EEG patterns change regularly while was induced with fentanyl (2.0 μg/kg), propofol (2.5 mg/kg), and entropy decreases. Because EEG-based monitoring systems cal- rocuronium (0.6 mg/kg). Anesthesia was maintained with propofol culate an index that reflects the hypnotic component of anest- continuous IV infusion under N2O in O2. -

A Comparative Study Between Entropy and Clinical Response To
Med. J. Cairo Univ., Vol. 87, No. 3, June: 2025-2031, 2019 www.medicaljournalofcairouniversity.net A Comparative Study between Entropy and Clinical Response to Determine the Requirement of Propofol for Induction of General Anesthesia in Geriatric Patients MAI A.K. NIDA, M.Sc.; WESAM F. MOUSA, M.D.; AHMED S. ELGEBALY, M.D. and HESHAM E. EL-ASHRY, M.D. The Department Anesthesiology and Surgical Intensive Care, Faculty of Medicine, Tanta University Abstract Key Words: Entropy – Clinical response – Requirement of propofol – Geriatric patients. Background: Anesthesia under dosage causes awareness, while over dosage results in drug complications. Endotracheal intubation must be after an adequate level of anesthesia. Introduction Propofol is an IV short-acting anesthetic which causes hypo- tension in a dose dependent effect. With entropy, SE level 40- ANESTHESIA under dosage causes awareness, 60 and RE-SE difference <10 are the target during anesthesia. while over dosage results in drug complications Geriatrics are different in EEG spectral patterns than young [1] patients and are more vulnerable to complications of endotra- . Endotracheal intubation must be after an ade- cheal intubation and propofol. quate level of anesthesia as light anesthesia leads to sympathetic stimulation, laryngospasm, bron- Aim of Study: The aim of this study was to compare chospasm, and traumatization of airway and over between the conventional clinical end-point of hypnosis and entropy on the dosage requirement of propofol and hemody- dosage of anesthetics with delay in intubation leads namic during propofol induction of general anesthesia in to bradycardia, arrhythmia, hypotension and at geriatric patients. last, respiratory and cardiovascular arrest [2] . -

Taylor V. Crawford
Case 2:05-cv-04173-FJG Document 202-1 Filed 07/24/2006 Page 1 of 20 IN THE UNITED STATES DISTRICT COURT WESTERN DISTRICT OF MISSOURI CENTRAL DIVISION MICHAEL ANTHONY TAYLOR ) ) Plaintiff, ) ) v. ) No. 05-4173-CV-W-FJG ) LARRY CRAWFORD, et al., ) ) Defendants. ) ____________________________________) PLAINTIFF’S OPPOSITION TO DEFENDANTS’ PROPOSED PROTOCOL JOHN WILLIAM SIMON DONALD B. VERRILLI, JR. J.D., PH.D. MATTHEW S. HELLMAN GINGER D. ANDERS ERIC BERGER 2683 South Big Bend Blvd, # 12 JENNER & BLOCK LLP St. Louis, Missouri 63143 601 13th Street NW (314) 645-1776 Washington, DC 20005 FAX: (314) 645-2125 (202) 639-6000 FAX: (202) 661-4983 Counsel for Plaintiff Case 2:05-cv-04173-FJG Document 202-1 Filed 07/24/2006 Page 2 of 20 TABLE OF CONTENTS TABLE OF AUTHORITIES .......................................................................................................... ii INTRODUCTION ...........................................................................................................................1 I. THE STATE’S PROPOSED PROTOCOL MUST BE REJECTED. .................................2 A. Monitoring Anesthetic Depth. .................................................................................2 B. IV Access.................................................................................................................5 C. Mixing the Chemicals..............................................................................................7 D. Drug Administration. ...............................................................................................8 -

New Indications for Peripheral Pulse Wave Acta Universitatis Tamperensis 2408
JARKKO HARJU New Indications for Peripheral Pulse Wave JARKKO HARJU New Indications for Peripheral Pulse Wave Acta Universitatis Tamperensis 2408 JARKKO HARJU New Indications for Peripheral Pulse Wave AUT 2408 AUT JARKKO HARJU New Indications for Peripheral Pulse Wave ACADEMIC DISSERTATION To be presented, with the permission of the Faculty Council of the Faculty of Medicine and Life Sciences of the University of Tampere, for public discussion in the auditorium of Finn-Medi 5, Biokatu 12, Tampere, on 14 September 2018, at 12 o’clock. UNIVERSITY OF TAMPERE JARKKO HARJU New Indications for Peripheral Pulse Wave Acta Universitatis Tamperensis 2408 Tampere University Press Tampere 2018 ACADEMIC DISSERTATION University of Tampere, Faculty of Medicine and Life Sciences Tampere University Hospital, Department of Anaesthesia Finland Supervised by Reviewed by Professor Arvi Yli-Hankala Docent Vesa Kontinen University of Tampere University of Turku Finland Finland Professor Niku Oksala Professor Tarmo Lipping University of Tampere Tampere University of Technology Finland Finland The originality of this thesis has been checked using the Turnitin OriginalityCheck service in accordance with the quality management system of the University of Tampere. Copyright ©2018 Tampere University Press and the author Cover design by Mikko Reinikka Acta Universitatis Tamperensis 2408 Acta Electronica Universitatis Tamperensis 1918 ISBN 978-952-03-0821-6 (print) ISBN 978-952-03-0822-3 (pdf) ISSN-L 1455-1616 ISSN 1456-954X ISSN 1455-1616 http://tampub.uta.fi Suomen Yliopistopaino Oy – Juvenes Print Tampere 2018 441 729 Painotuote To Eeva, Anna and Olli List of original publications This thesis is based on the following four original publications, referred to in the text by their Roman numerals (I-IV): I Matthias Gruenewald, M.*, Harju, J.*, Preckel, B., Molnár, Z., Yli- Hankala, A., Roßkopf, F., Koers, L., Orban, A., Bein, B. -

Adequacy of Anaesthesia (Aoa) Clinical Brochure Adequacy of Anaesthesia (Aoa)
GE Healthcare Adequacy of Anaesthesia (AoA) Clinical brochure Adequacy of Anaesthesia (AoA) AoA parameters provide continuous non-invasive measurement of: Adequacy of Anaesthesia (AoA) is a • Depth of anaesthesia with SPECTRAL ENTROPY™ concept made up of various parameters • Patient’s response to surgical stimuli and analgesic medications with SURGICAL PLETH INDEX (SPI)™ to help you assess patients’ individual • Muscle relaxation/recovery with NEUROMUSCULAR responses to the delivery of inhaled TRANSMISSION (NMT) and intravenous hypnotics, opioids, and The AoA split screen view incorporates values and trends obtained from the SPI, Entropy (State Entropy SE, Response Entropy RE and NMBAs during general anaesthesia. Burst Suppression Ratio BSR) and Neuromuscular Transmission (NMT) modules, providing a holistic view of the patient’s response to anaesthesia. When seconds counts in an intensive and multitasking enviroment, the BalanceView is guidance for prompt visualization of the patients’ responses to changes of anaesthesia conditions and may help save valuable time on responsiveness to analgesia/depth of anaesthesia optimization for each individual patient. The “white dot” that moves drastically away from the target zone may indicate inadequate hypnosis or analgesia level. Target Region Level of conciousness of Level Nociception-Antinociception Balance. Current data support SPI target range 20-50. Target at present reflects only the user preference. Spectral Entropy™ Control Entropy Entropy monitoring provides two indexes: parameters such as the EEG raw signal The GE Healthcare Entropy module, Figure 1 - Propofol consumption μg/kg/min • State Entropy (SE): Steady and robust which can help for in depth and more 120 E-ENTROPY, and accessories are -9% signal. The State Entropy value is always comprehensive analysis of state of brain. -

Powering Your Performance
POWERING YOUR PERFORMANCE B105M | B125M | B155M Modular Patient Monitors gehealthcare.com Advanced capabilities. Simple scalability. Integrated technology. B1x5M, B125M and B155M represent an additional solution to GE Healthcare’s monitoring platforms. Clinical precision It delivers a premium clinical performance across various care areas inherited from decades of research and innovation done with customer support. Scalable platform With B1x5M, you can monitor essential vital signs like ECG, SpO , Temperature, Non Invasive Blood Pressure, 2 Intuitive Respiration and you can also easily scale up your design monitoring capabilities using modules to monitor with precision respiratory gases and anesthetic agents, NMT and Entropy™ – with up to three advanced Integrated parameters simultaneously. technologies These precise, trustworthy, and easy-to-use monitors enable simple and Intuitive workflows with a choice of 10-, 12- or 15- inch touch screen displays to address your clinical and workflow related challenges. Legacy of premium clinical performance. Excellence of advanced features. Because every heartbeat counts… With decades of expertise designing The B1x5M range of modular patient monitors helps you quickly manage patient changing advanced and proven clinical algorithms conditions like arrhythmias and high/low blood pressure, and efficiently assess the level of and measurement technologies, this consciousness. range of monitors benefits parameter These monitors seamlessly integrate with your CARESCAPE Ecosystem to centralize patient innovations. It features: data, alarms, and to automatically capture and document vital signs. It decreases manual tasks, unnecessary movements, and as a result, improving workflows. • DINAMAP™ B1x5M range allows you to effectively monitor deteriorating patient conditions and make • SuperSTAT™ timely interventions by tracking the National Early Warning Score (NEWS). -

Adequacy of Anesthesia Guidance for Colonoscopy Procedures
pharmaceuticals Article Adequacy of Anesthesia Guidance for Colonoscopy Procedures Michał Jan Stasiowski 1,2,* , Małgorzata Starzewska 3, Ewa Niewiadomska 4, Seweryn Król 2,5 , Kaja Marczak 2, Jakub Zak˙ 1,2, Aleksandra Pluta 1,2, Jerzy Eszyk 6, Beniamin Oskar Grabarek 7,8 , Izabela Szumera 1,2, Michał Nycz 5 , Anna Missir 2, Lech Krawczyk 1 and Przemysław Jałowiecki 1 1 Department of Emergency Medicine, Faculty of Medical Sciences in Zabrze, Medical University of Silesia, 40-555 Katowice, Poland; [email protected] (J.Z.);˙ [email protected] (A.P.); [email protected] (I.S.); [email protected] (L.K.); [email protected] (P.J.) 2 Department of Anaesthesiology and Intensive Therapy, 5th Regional Hospital, Medykow Square 1, 41-200 Sosnowiec, Poland; [email protected] (S.K.); [email protected] (K.M.); [email protected] (A.M.) 3 Department of Cardiac Anaesthesia and Intensive Care, Silesian Centre for Heart Diseases, 41-800 Zabrze, Poland; [email protected] 4 Department of Epidemiology and Biostatistics, School Health Sciences in Bytom, Medical University of Silesia, Piekarska 18 Street, 41-902 Bytom, Poland; [email protected] 5 Department of General, Colorectal and Polytrauma Surgery, Faculty of Health Sciences in Katowice, Medical University of Silesia, 40-555 Katowice, Poland; [email protected] 6 Unit of Endoscopy by the Department of Gastroenterology, 5th Regional Hospital, Medykow Square 1, 41-200 Sosnowiec, Poland; [email protected] 7 Department of Histology, Cytophysiology and Embryology, Faculty of Medicine -

Monitoring of Depth of Anesthesia Using Entropy Monitor During Cardiopulmonary Bypass 1Pragya Sachan, 2Naina P Dalvi
RIA Monitoring of Depth10.5005/jp-journals-10049-0032 of Anesthesia using Entropy Monitor ORIGINAL RESEARCH Monitoring of Depth of Anesthesia using Entropy Monitor during Cardiopulmonary Bypass 1Pragya Sachan, 2Naina P Dalvi ABSTRACT How to cite this article: Sachan P, Dalvi NP. Monitoring of Depth of Anesthesia using Entropy Monitor during Cardiopulmonary To monitor the depth of anesthesia using entropy monitor Aim: Bypass. Res Inno in Anesth 2017;2(2):45-50. during cardiopulmonary bypass (CPB) using (1) numerical indices provided by entropy monitor [response entropy (RE) Source of support: Nil and state entropy (SE), burst suppression ratio (BSR)] and (2) Conflict of interest: None modified version of Brice questionnaire used after 48 hours of surgery. Materials and methods: Design: Prospective observational INTRODUCTION study. Setting: Cardiovascular thoracic surgery (CVTS) opera- General anesthesia is defined as the condition of loss of tion theater (OT) of our hospital. Participants: 100 patients consciousness where patient is unresponsive to painful undergoing CPB surgery and willing to participate in the study. 1 Intervention: Brain and facial muscular activity recorded using surgical stimuli. However, patients do recall various disposable sensor with three electrodes attached to patient’s experiences during the surgery, known as “invisible scars forehead and a sensor cable that connects the sensor to entropy of the surgery.” The principle of entropy is that increas- module. Entropy indices were calculated and displayed in real ing depth of anesthesia causes increase in regularity of time. All patients were then interviewed 48 hours after surgery the electroencephalogram (EEG), which is then inferred about any recall of intraoperative awareness using the modified by entropy and used to estimate the depth of anesthesia. -

Monitoring the Depth of Anaesthesia Using the New Modified Entropy Sensors During Supratentorial Craniotomy: Our Experience
Original Article Monitoring the depth of anaesthesia using the new modified entropy sensors during supratentorial craniotomy: Our experience Richa Sharma, Pirjo Manninen, Lashmikumar Venkatraghavan Abstract Background: Monitoring the depth of anaesthesia can be a challenge in patients undergoing supratentorial craniotomy because the conventional sensors for both bispectral index and entropy monitors lose their contact with a brain after scalp elevation. The new sensors for the entropy monitor are more flexible and can be placed in different locations. The purpose of this study was to determine the feasibility on the use of new GE entropy sensors in monitoring depth of anaesthesia in patients undergoing supratentorial craniotomy. Materials and Methods: We retrospectively reviewed the data from 20 consecutive patients undergoing supratentorial craniotomy who had the monitoring of the depth of anaesthesia using modified entropy sensors. Prior to the induction of anaesthesia, the new GE entropy sensor (P/N M1038681) was applied in a modified fashion. We measured the state entropy (SE) and response entropy (RE) at 12 perioperative time points. Entropy values were compared with the clinical indices of depth of anaesthesia. Results: Data from 20 consecutive patients (orbitozygomatic craniotomy [10] and bifrontal craniotomy [10]) were analysed. Monitoring was possible in all the patients. The changes in entropy values correlated with clinical indices of depth of anaesthesia. However, some patients showed variations in absolute values (RE and SE) during the intraoperative period without any changes in the level of anaesthetic depth. Conclusions: Monitoring the depth of anaesthesia is feasible with the use of new entropy sensors in patients undergoing supratentorial craniotomy. In contrast to standard sensors, the new sensors offer flexibility with the placement. -

Monitoring the Depth of Anaesthesia
Sensors 2010, 10, 10896-10935; doi:10.3390/s101210896 OPEN ACCESS sensors ISSN 1424-8220 www.mdpi.com/journal/sensors Review Monitoring the Depth of Anaesthesia Bojan Musizza 1 and Samo Ribaric 2,* 1 Department of Systems and Control, Jožef Stefan Institute/Jamova 39, SI-1000 Ljubljana, Slovenia; E-Mail: [email protected] 2 Institute of Pathophysiology, Faculty of Medicine, University of Ljubljana/Zaloška 4, SI-1000 Ljubljana, Slovenia * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +386-1-543-70-53; Fax: +386-543-70-21. Received: 1 September 2010; in revised form: 29 September 2010 / Accepted: 22 November 2010 / Published: 3 December 2010 Abstract: One of the current challenges in medicine is monitoring the patients’ depth of general anaesthesia (DGA). Accurate assessment of the depth of anaesthesia contributes to tailoring drug administration to the individual patient, thus preventing awareness or excessive anaesthetic depth and improving patients’ outcomes. In the past decade, there has been a significant increase in the number of studies on the development, comparison and validation of commercial devices that estimate the DGA by analyzing electrical activity of the brain (i.e., evoked potentials or brain waves). In this paper we review the most frequently used sensors and mathematical methods for monitoring the DGA, their validation in clinical practice and discuss the central question of whether these approaches can, compared to other conventional methods, reduce the risk of patient awareness during surgical procedures. Keywords: consciousness; cognitive binding; general anaesthesia monitors; soft sensors; general anaesthesia Sensors 2010, 10 10897 1.