Assessment of Ground Water Quality in Different Areas of Hyderabad
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1. DSNR to VNR VJIET: Rs
TRANSPORT for Senior (II, III, IV) B.Tech and M.Tech Students - Exclusive RTC Buses -2021-22 Academic year For the convenience of the students, arrangements have been made with the TSRTC to run the following exclusive buses to the Institute from different places of city. Sl No From To Fare per year Rs 1 Dilshukhnagar VNRVJIET 17,100-00 2 Kachiguda VNRVJIET 15,600-00 3 Uppal VNRVJIET 15,600-00 4 Kondapur VNRVJIET 12,600-00 5 BHEL VNRVJIET 9,000-00 6 Kukatpally VNRVJIET 8,200-00 1. DSNR to VNR VJIET: Rs. 17,100-00 Bus Timings : DSNR Dep : 8:10 am--- VNR VJIET Arrival : 9:40am VNR VJIET Dep : 4:35 pm --- DSNR Arrival : 6:05 pm Bus Route : Dilsukhnagar ( Chandana Bros) – Moosaram Bagh – Malakpet – Nalgonda X roads-Chadarghat - Womens College – Osmania Medical College – Abids – Nampally – Lakadikapool – Khairatabad – Erramanzil – NIMS – Panjagutta Colony – Ameerpet – Maitrivanam – S.R. Nagar – ESI - Erragadda – Bharatnagar – Moosapet – VNR VJIET (via – Pragathi Nagar) 2. Kachiguda – VNR VJIET: Rs. 15,600-00 Bus Timings : Kachiguda Dep : 8:00 am -------- VNR VJIET Arrival : 9:40 am VNR VJIET Dep : 4:35 pm -------- Kachiguda Arrival : 6:00 p Bus Route : Kachiguda Rly Stn – Bharkatpura – PF Office – Fever Hospital – Nallakunta – Shanker Mutt – VST – RTC x Roads – Musheerabad - Golconda x Roads – Boiguda – Chilakalguda – Secunderabad Stn ( Rethifile) – Pass Port Office – Patny ( MCH ) Race Course – Tadbund – New Bowenpally – Air Force Station – Balanagar – Moosapet – VNR VJIET (via – Pragathi Nagar) 3. Uppal – VNR VJIET: Rs. 15,600-00 Bus Timings : Tarnaka Dep : 8:10 am -------- VNR VJIET Arrival : 9:40 am VNR VJIET Dep : 4:35 pm -------- Tarnaka Arrival : 6:00 pm Bus Route : Tarnaka ( Fly Over) , Mettuguda – Chilakalaguda - Secunderabad Stn (Rethifile) , Pass Port Office- Patny ( MCH Office ) – Anand Theatre - Begumpet Police lines - Prakash Ngr - Shamlal Building – Life Style – Green Lands – Ameerpet – Maitrivanam – S.R. -

(Sq.Mt) No of Kits Madan Kumar Karana
Name of the house owner Area available on No of S.No House number & Location Sri/Smt terrace (Sq.mt) kits Flat No:101,Plot No: 28, Sri Lakshmi Nilayam, Krishna 100 Sq.mt 1 Madan Kumar Karanam 1 kit Nagar colony, Near Gandhian School, Picket, Sec’bad 4th floor 2 K.Venkateshwar 4-7-12/46A,Macharam, Ravindranagar, Hyd 400 sft 1 kit No:102, Bhargav residency, Enadu colony, 3 N.V.Krishna Reddy 2500 sft 4th floor 1kit Kukatpally,Hyderabad 500 sft 4 Ghous Mohiuddin 5-6-180,Aghapura, Hyd 1 kit 2nd floor 5 Abdul Wahed 18-1-350/73,Yousuf bin colony,chandrayangutta,Hyd 1 kit 260, Road no: 9B,Alkapuri, 6 Cherukupalli Narasimha Rao 1200 sft 1 kit Near sai baba temple 10-5-112,Ahmed Nagar, Masab tank, 7 Ahmed Nizamuzzana Quraishi Rs.3000 sft 1 kit Hymayunangar,yderabad 9-7-121/1, Maruthi nagar, Opp:Santhosh Nagar 8 K.Vjai Kumar 100 sft 1 kit colony Saidabad, Hyd 9 B. Sugunakar 8-2-121, Behind Big Bazar, Punjagutta, Hyd 900 sft 1 kit 10 D. Narasimha Reddy 3.33.33 LV Reddi colony, Lingampally, Hyd 900 sft 1 kit 1-25-176/9/1, Rahul enclave, Shiva nagar, 11 D. Radhika 1000 sft 1 kit Kanajiguda, Trimalgherry, Secunderabad 2-3-800/5, Plot no: D-4,Road no:15,Co-op bank 12 B. Muralidhara Gupta 1600 sft 1 kit colony, Nagole, Hyderabad 13 Dr.Mazar Ali 12-2-334/B,Murad nagar, Mehdipatnam,Hyderabad 1600 sft 2 kits 14 Sukhavasi Tejorani 22-32/1, VV nagar, Dilshukhnagar, Hyd 800 sft 1 kit 15 Induri Bhaskara Reddy MIG 664, Phase I & II, KPHB colony,Kukatpally, Hyd 1000 sft 1 kit No-18, Subhodaya nagar colony, near HUDA park, 16 T.Sundary 150 yards 1 kit Opp: KPHB, Kukatpally, Hyd. -

Date of AGM(DD-MON-YYYY) 28-JUL-2017
Note: This sheet is applicable for uploading the particulars related to the unclaimed and unpaid amount pending with company. Make sure that the details are in accordance with the information already provided in e-form IEPF-2 CIN/BCIN L85195TG1984PLC004507 Prefill Company/Bank Name DR.REDDY'S LABORATORIES LTD Date Of AGM(DD-MON-YYYY) 28-JUL-2017 Sum of unpaid and unclaimed dividend 0.00 Sum of interest on matured debentures 1412295.50 Sum of matured deposit 0.00 Sum of interest on matured deposit 0.00 Sum of matured debentures 0.00 Sum of interest on application money due for refund 0.00 Sum of application money due for refund 0.00 Redemption amount of preference shares 0.00 Sales proceed for fractional shares 0.00 Validate Clear Proposed Date of Investor First Investor Middle Investor Last Father/Husband Father/Husband Father/Husband Last DP Id-Client Id- Amount Address Country State District Pin Code Folio Number Investment Type transfer to IEPF Name Name Name First Name Middle Name Name Account Number transferred (DD-MON-YYYY) ARUN KUMAR RADHAKRISHAN A-40,(60.MEATER),POCKET-00,,SECTOR-2,ROHINI,DELHIINDIA Delhi Delhi FOLIOA00001 Interest on matured debentures 33.30 23-MAR-2019 BALVEER SINGH BHADOURIA LATE SRIGS BHADO A-905/3,MIG SECTT.A,MAZAR ROAD,INDIRAINDIA NAGAR,,LUCKNOW,UTTAR PRADESH LUCKNOW FOLIOB00217 Interest on matured debentures 199.80 23-MAR-2019 B VSREE KRISHNA MADHUSUDANA RAOB V 101, VIKASINI APTS,2-2-1121/3 & 3A,NEWINDIA NALLAKUNTA,HYDERABADTELANGANA HYDERABAD FOLIOB00594 Interest on matured debentures 55.50 23-MAR-2019 -

Annual Report 2018-2019 Contents
ANNUAL REPORT 2018-2019 ANNUAL REPORT 2018-2019 CONTENTS Notice President’s Message Board of Directors Report Managing Committee MC Meeting Details for the year 2018-2019 Other Committees Training Programs organized During 2018-2019 Important Events during 2018-2019 ALEAP Credit Guarantee Association (ACGA) Center for Entrepreneurs Development (CED) Atal Incubation Centre ALEAP Women Entrepreneurs Hub (AIC ALEAP We-Hub) Women Entrepreneurs International Trade & Technology Hub (WITH) ALEIZONE Auditor’s Report & Balance Sheet for the year 2018-2019 ANNUAL REPORT 2018-2019 NOTICE Notice is hereby given that 25th Annual General Meeting of Association of Lady Entrepreneurs of India (ALEAP) will be held on Monday, 30th September, 2019 at 02.00 P.M at ALEAP Industrial Estate, Gajularamaram, Pragathi Nagar Road, OPP: JNTU, Kukatpally, Hyderabad, R.R. District to transact the following Businesses: ORDINARY BUSINESS: 1. To receive, consider and adopt the audited accounts of the company for the year ended 31st March, 2019 and the reports of the Directors and Auditors thereon. 2. Auditor’s Appointment To consider and if thought fit, to pass, with or without modification, the following resolution as an ordinary Resolution: “RESOLVED THAT pursuant to the provisions of Section 139(1) and all other applicable provisions of the Companies Act, 2013, and the Rules made there under, M/s. Nataraja Iyer and Co (having ICAI Firm Regn No. 002413S) , Chartered Accountants, Hyderabad who have offered themselves for re-appointment and have confirmed -

Lohitha Residency
https://www.propertywala.com/lohitha-residency-hyderabad Lohitha Residency - Pragathi Nagar, Hyderabad offers great lifestyle Lohitha Residency by Lohitha Infra Projects Private Limited at Pragathi Nagar, Bachupally in Hyderabad offers residential project that host 2 bhk apartment in various sizes. Project ID: J811901448 Builder: Lohitha Infra Projects Private Limited Location: Lohitha Residency,Bachupally, Pragathi Nagar, Hyderabad (Telangana) Completion Date: Dec, 2020 Status: Started Description Lohitha Residency presents 2 BHK Apartments in Pragathi Nagar, Bachupally, Hyderabad. The size of the apartment may ranges from 868 to 1250 sqft. With various amenities like gym, car parking, water supply etc for the residents. With well-ventilated apartments and uncluttered nature space.Project is to provide unmatched connectivity from all the important landmarks and places of everyday utility such as hospitals,schools, super marts, parks, etc. Amenities: Lift Power backup Security Lohitha Infra Projects Private Limited is a Private incorporated on 14 October 2008. It is classified as Non-govt company and is registered at Registrar of Companies, Hyderabad. Its authorized share capital is Rs. 1,000,000 and its paid up capital is Rs. 100,000. It is inolved in Real estate activities with own or leased property. Features Luxury Features Security Features Lifts High Speed Internet Security Guards Lot Features Interior Features Balcony Feng Shui / Vaastu Compliant Exterior Features Recreation Reserved Parking Fitness Centre / GYM Club / Community -
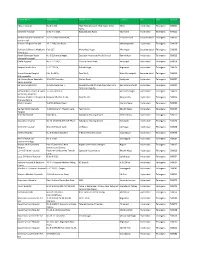
RHICL Network Hospital List.Xlsx
Hospital Name Address Line 1 Address Line 2 Location City State Pincode Aditya Hospital No.4-1-160, Tilak Road,Opposite Tilak Nagar Water Abids Hyderabad Telangana 500001 Tanks, Kamineni Hospital D.No.4-1-1227, Boggulakunta Road, King Kothi Hyderabad Telangana 500001 Krishna Institute Of Medical 1-8-31/1 Ministers Road, Secunderabad Secunderabad Telangana 500003 Sciences Ltd Premier Hospitals Pvt Ltd 12-2-718,Cross Road, Mahedipatnam Hyderabad Telangana 500028 Rainbow Children's Medicare Plot C17, Manovikas Nagar, Vikrampuri Secunderabad Telangana 500009 Private Ltd. Advalli Damodar Reddy No.9-2,Sharada Nagar, Opposite Hyderabad Public School, Ramanthpur Hyderabad Telangana 500013 Memorial Hospital Challa Hospital No.7-1-71/A/1, Dharam Karan Road, Ameerpet Hyderabad Telangana 500016 Swapna Health Care 6-3-1111/19, Nishath Bagh, Begumpet Hyderabad Telangana 500016 Basant Sahney Hospital Plot No 29/A, Road No.1, West Marredpally Secunderabad Telangana 500026 Multispeciality Sai Krishna Super Speciality 3-51,VLR Complex, Station Road, Kachiguda Hyderabad Telangana 500027 Neuro Hospital Sai Vani Hospital Ltd. 1-2-365/36/6 And 7, Ramakrishna Mutt Road,Opposite Indira Ramakrishna Mutt Hyderabad Telangana 500029 Park,Domalaguda, Sathya Kidney Centre & Super 3-6-426,Street-4, Himayath Nagar Hyderabad Telangana 500029 Speaciality Hospitals Rainbow Children's Hospital & Banjara Hills,Plot No 22, Road No 10, Banjara Hills Hyderabad Telangana 500034 Prenatal Centre Mythri Hospital 5-4/12-16,Main Road, Chanda Nagar Hyderabad Telangana 500050 Sai Ram -

List of Unclaimed Dividend for the Financial Year 2017-18 As on 01-July-2021
LIST OF UNCLAIMED DIVIDEND FOR THE FINANCIAL YEAR 2017-18 AS ON 01-JULY-2021 SL NO MEM.NO NAME SRI / SMT / M/S: ADDRESS 1 1 SRI B.N.RATHI 4-5-173,SULTAN BAZAR,HYD,500002 2 30 HUKUMCHAND BHANGADIA 18-4-50,SHAMSHEER GUNJ,HYD,500053 3 31 HARIKISHAN LAHOTI 18-4-50,SHAMSHEER GUNJ,HYD,500053 4 41 NIRMALA SABOO "MANISHA",EDEN BAGH,HYD,500001 5 45 R V PAHADE 3-5-141,EDEN BAGH RAMKOTE,HYD,500001 6 46 KALAVATI DHOOT GOPAL BHAVAN,BASHEER BAGH POLICE COMP,HYD,500029 7 56 NARSING DAS JHAWAR H.NO.12-8-12,IST FLOOR,HUNTER ROAD,RAMANNAPET,WGL,506002 8 63 PRATIBHA MAHESHWARI 402, ROAD NO 5,BANJARA HILLS,HYD,500034 9 73 SHARAYU BAJAJ 4-1-1011,BAGULKUNTA,HYD,500001 10 84 RAJKUMARI SARDA C/o UNITED STEELS,10/11 PAN BAZAR,SEC-BAD,SEC,500003 11 113 GITA BAI SONI 11-3-949,MALLE PALLY,HYD,500001 12 114 MOTILAL SONI NO.11-3-949,MALLAPALLY,HYD,500001 13 118 URMILA BHANDARI 15/7/160/2 LAXMANGIRI,MATH BEGUM BAZAR,HYD,500012 14 120 MANKANVER CHANDAK 10-2-196,EAST MARREDPALLY,SEC-BAD,SEC,500026 15 122 KAMLA DEVI JAJU 1805,MAHANKALI STREET,SEC-BAD,SEC,500003 16 128 BALA PRASAD MUNDHADA 12-11-205/1,WARISGUDA,SEC-BAD,SEC,500061 17 130 SHIVANATH LADHA 12-11-331,WARISGUDA,SEC-BAD,SEC,500061 18 131 NANDKISHORE BUNG PLT.NO.16,RADHE SWAMY COLONY,SIKH ROAD,BOWENPALLY,SEC,500009 19 135 USHADEVI BAHETI 8-2-171 TURNER STREET,BEHIND EMMANUEL PH STUDIO,SEC-BAD,SEC,500003 20 136 CHTRABHUJ CHANDAK 6-1-606,KHAIRATABAD,HYD,500004 21 160 RAM NIVAS SARDA SHAH GUNJ,SHAH GUNJ,HYD,500064 22 163 RAMPERSHAD BANG S/O KHAJULAL BANG,2-4-1081 KACHIGUDA,HYD,500027 23 175 MURLIDHAR LOYA MAIN ROAD,BELLAM -

7.25 102 HCL Colony 7.33 103 ECIL 7.40 104 AS RAO Nagar 7.46 105 R
ROUTE NO - 1 CHARLA PALLY B P NO BOARDING POINT TIME 101 CHARLA PALLY ( Village) 7.25 102 HCL Colony 7.33 103 ECIL 7.40 104 A.S. RAO Nagar 7.46 105 R.K.PURAM 8.00 106 LAL BAZAR 8.10 107 JANAPRIYA COMPLEX 8.20 108 SELECT TALKIES 8.25 109 BOLLARAM BAZAR 8.28 CMR College Ground 8.50 CMR Technical Campus VEHICLE NO: AP 28TD 5213 SEATING CAPACITY 55 Incharge B.Vasantha Lakshmi Ph No:9908620218 Driver Venkataiah Ph No:9704616558 Owner Raj Reddy Ph No:9397333222 College CMR CET ROUTE NO - 2 RAMPALLY X Roads B P NO BOARDING POINT TIME 201 RAMPALLY X Roads 7.25 202 KANDI GUDA CIRCLE 7.27 203 AYAPPA COLONY 7.30 204 DAMAIGUDA 7.35 205 JAMMAIGUDDA 7.40 206 YELLA REDDY GUDA 7.43 207 LIFELINE Hospital 7.47 208 RADHIKA TALENT SCHOOL 7.49 209 KANDI GUDA CIRCLE 7.50 210 YAPRAL BUS STOP 8.00 CMR College Ground 8.50 CMR Technical Campus VEHICLE NO: AP29T2856 SEATING CAPACITY 49 Incharge G.Krishna Lava Kumar Ph No:8985676607 Driver SATISH Ph No:9963298136 Owner Raj gopal reddy Ph No:9848359336 College CMR EC ROUTE NO - 3 NAGARAM B P NO BOARDING POINT TIME 301 NAGARAM 7.35 302 VIJAYA HOSPITAL 7.37 303 CHAKRI PURAM (Bus Stop) 7.39 304 NAGARJUNA NAGAR(Bus Stop) 7.40 305 KUSHAIGUDA(Bus Stop) 7.42 306 SHOPPING COMPLEX(Sainikpuri) 7.50 307 NIRMAL NAGAR 7.52 308 CDM 7.55 CMR COLLEGE GROUND 8.50 CMR TECHNICAL CAMPUS VEHICLE NO: KA01AF 0099 SEATING CAPACITY 54 Incharge D.Dev Singh Ph No:9848361255 Driver Bhaskar Ph No:9849484105 Owner PRATAP REDDY Ph No:9000197909 College CMRIT ROUTE NO - 4 SAI NAGAR (Saket) B P NO BOARDING POINT TIME 401 SAI NAGAR (Saket) 7.30 402 -

Hospital List.Xlsx
Sr. No. Name of Insurance Co. State City Provider Name Category Address Pin Code Tel Area Tel No. Fax No. Not Servicing to Provider Code Code Insurance Co., but No. 1 PSU-United India Insurance Company Andhra Anantapur AASHA HOSPITAL(APOLLO HOSPITAL- Hospital Door No 7/201,Court Road,Anantapur 515001 08554 274194/237818 245755 2463 Pradesh ANANTAPUR) 2 PSU-United India Insurance Company Andhra Anantapur DR. Y.S.R. MEMORIAL HOSPITALS Hospital # 12-2-878, 1# Cross,,,Sai Nagar, 515001 08554 232727 / 247365 10710 Pradesh 3 PSU-United India Insurance Company Andhra Anantapur SRI PRAKASH EYE HOSPITAL Hospital # 12-3-216, Sai Nagar, Main Road,,5th Croos,,Beside 515001 08554 221006 245906 75521 Pradesh Veternary Hospital, 4 PSU-United India Insurance Company Andhra Anantapur VASAN EYE CARE Hospital # 15/581, Street Raju Road,,Anantapur 515001 08554 222445 / 302100 302199 14095 Pradesh HOSPITAL(ANANTAPUR) 5 PSU-United India Insurance Company Andhra Bhimavara BHIMAVARAM HOSPITAL Hospital J P Road,,West Godavari,, 534204 08816 221111 / 22 / 33 221100 3903 Pradesh m 6 PSU-United India Insurance Company Andhra Chittoor ARAGONDA APOLLO HOSPITALS Hospital Aragonda Village,,Thavanam Palli Mandal,,Chittoor 517129 08573 283221 / 283222 283223 4504 Pradesh 7 PSU-United India Insurance Company Andhra Chittoor SREELATHA MODERN EYE HOSPITAL & Hospital # 2-63 /1, Officers Lane,,Beside Municipal 517001 08572 226660 / 222121 / 233391 233351 5028 Pradesh RESEARCH CENTRE Office,,Chittoor 8 PSU-United India Insurance Company Andhra East APOLLO SAMUDRA Hospital 13-1-3, Main Road,Near 2 Town Police 533001 0884 2345700/2345800/900 2379141 2806 Pradesh Godavari HOSPITALS(KAKINADA) Chowky,Kakinada 9 PSU-United India Insurance Company Andhra East SWATANTRA HOSPITALS PVT.LTD. -
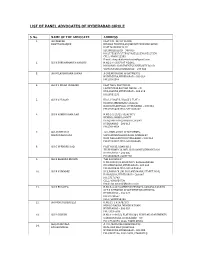
Advocate List with Phone Numbers
LIST OF PANEL ADVOCATES OF HYDERABAD CIRCLE S. No. NAME OF THE ADVOCATE ADDRESS 1. SRI DEEPAK FLAT 101, 38, IST FLOOR BHATTACHARJEE NIRMAN TOWERS,ADJ.SHENOY NURSING HOME EAST MARREDPALLY SECUNDERABAD – 500 026 PH:27732657/27730174,65211550/65217550 CELL: 98490 22303 E-mail: [email protected] 2. SRI K SUBRAHMANYA NARUSU H.NO.2-1-233,FLAT NO.002, MANOHAR APARTMENTS,UNIVERSITY ROAD VIDYANAGAR,HYDERABAD – 500 044 3. SRI M LAKSHMANA SARMA A-205,MATRUSRI APARTMENTS HYDERGUDA,HYDERABAD – 500 029 PH:23545599 4. SRI D L KIRAN PRAKASH FLAT NO.6, PLOT NO.36 LAXMINIVAS,KALYAN NAGAR – III ERRAGADDA,HYDERABAD – 500 010 PH:23831231 5. SRI B S PRASAD HIG I, PHASE II, BLOCK I, FLAT 2 BEHIND AMBEDKAR COLLEGE BAGHLINGAMPALLI, HYDERABAD – 500 044 PH:27669025 CELL:9849024267 6. SRI K ASHOK RAMA RAO H.NO.2-2-23/22-18/A(397) BEHIND AHOBILA MATT DURGABHAI DESHMUKH COLONY HYDERABAD – 500 013 PH:27614654 7. SRI ADDEPALLI 104, HIMA SHIVA APARTMENTS, SURYANARAYANA SHIVAM MAIN ROAD,BAGH AMBERPET NEW NALLAKUNTA,HYDERABAD – 500 044 PH:27423287 CELL:9848055655 8. SRI G UPENDER RAO PLOT NO.35, ROAD NO.2 TELEPHONE COLONY, SRI RAMAKRISHNAPURAM HYDERABAD – 500 035 PH:24053008, 24037768 9. SRI B KRISHNA MOHAN "SRI SAI KRUPA" H.NO.180153/1,ROAD NO.5, KAMALANAGAR DILSUKHNAGAR,HYDERABAD – 500 660 PH:24048040 CELL:98493 86564 10. SRI B N SWAMIJI 513, B-BLOCK ,SRI SAI LAND MARK, STREET NO.8, HABSIGUDA,HYDERABAD – 500 007 PH: 27176743 CELL: 9391036739 Email: [email protected] 11. SRI K BUSAPPA H.NO.3-4-247/A,BEHIND VENKATA RAMANA TALKIES OPP.R K BHAVAN APARTMENTS,KACHIGUDA HYDERABAD – 500 027 PH: 27732657 CELL: 98483 98196 12. -

Vallurupalli Nageswara Rao Vignana Jyothi Institute of Engineering And
VALLURUPALLI NAGESWARA RAO VIGNANA JYOTHI INSTITUTE OF ENGINEERING AND TECHNOLOGY An Autonomous Institute, NAAC Accredited with ‘A’ Grade NBA Accredited for CE, EEE, ME, ECE, CSE, EIE, IT B.Tech Courses Approved by AICTE, New Delhi, Affiliated to JNTUH Recognized as “College with Potential for Excellence” by UGC Estd.1995 Vignana Jyothi Nagar, Pragathi Nagar, Nizampet (S.O), Hyderabad – 500 090, TS, India. Telephone No: 040-2304 2758/59/60, Fax: 040-23042761 E-mail: [email protected], Website: www.vnrvjiet.ac.in DEPARTMENT OF COMPUTER SCIENCE AND ENGINEERING 2012-13 S.No Name of the Student H.T Number Name of Organisation Area of Internship Duration Academic 1 G.LAKSHMI HARSHITHA 09071A0577 Year 2 JUNUTHULA MONICA 09071A0581 BSNL Product Development 2–4 Months 2012-13 3 V.SAI DIVYA 09071A05B5 4 VINUTHNA GADDIPATI 09071A0555 AVD Services Pvt Ltd Product Development 2–4 Months 2012-13 5 CHIDRA SANTHOSH KUMAR 09071A0512 2 –4 Months 6 KONAKALLA HITESH BHARGAVA 09071A0532 Net Enrich Technologies Product Development 2012-13 7 S.JAGADISWAR REDDY 09071A0559 2–4 Months 8 A.PADMA DEEPKA 09071A0502 2–4 Months DRDO Product Development 2012-13 9 G.LAKSHMI ALEKHYA 09071A0516 10 M.SANIL RAJ CHOWHAN 09071A0570 11 M.GNANDEEP 09071A0588 2–4 Months NI-MSME Product Development 2012-13 12 S KARTHIK RAGHU VAMSI 09071A05A6 13 SATELLI HITESH 09071A05A7 14 AVITI SHIVANI 09071A0505 15 GANAPURAM RAJESH 09071A0517 2–4 Months ViTech Systems ASIA Product Development 2012-13 16 G.KIRAN KUMAR 09071A0518 17 K.SRIKANTH 10075A0506 18 ARRA SNEHA SRIVATSAV 09071A0504 -

CTS) for Hyderabad Metropolitan Area (HMA
Hyderabad Metropolitan Development Authority IMMEDIATE ACTION PLAN Comprehensive Transportation Study (CTS) for Hyderabad Metropolitan Area (HMA) www.ctshma2011.com Immediate Action Plan: Summary December 2011 LEA Associates South Asia Pvt., Ltd., New Delhi, India in Joint Venture with LEA International Ltd., Canada IMMEDIATE ACTION PLAN: Consultancy Services for Comprehensive Transportation Study (CTS) Summary for Hyderabad Metropolitan Area (HMA) Preface LEA Associates South Asia Pvt Ltd., India along with LEA International Ltd., Canada are commissioned by the Government of Andhra Pradesh through HMDA to conduct Comprehensive Transportation studies and prepare long term strategic transportation plan for Hyderabad Metropolitan Area in March 2011. As a part of the Comprehensive Transportation Studies, to address the problems relating to the following aspects are required to be studied and evolve measures to mitigate these problems. These measures are to provide relief in the short term and be less resource intensive. • Area Traffic Management (5 areas- 25 Sq.kms) • Traffic corridor Management (7 corridors-15 Kms) • Improvement of Intersections (30 Nos) • Traffic Improvement plan for a terminal (MGBS) • On Street parking facilities (6 corridors) • Off Street parking facilities (6 locations) • Pedestrians facilities (50km of road stretch) In selecting the areas, corridors and junctions, to be studied, considerable amount of consultations with stakeholders like GHMC, Traffic police, HMR, APSRTC were held. After intensive deliberations the locations for study were selected and field studies were conducted. Field studies included reconnaissance, inventory of roads, classified traffic volume counts at midblocks and intersections, parking accumulation and duration surveys, pedestrian counts, speed and delay studies etc. The data collected from field surveys was thoroughly analysed to identify and quantify the problems of traffic.