Aspen Physical Property Methods
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chapter 4 Calculation of Standard Thermodynamic Properties of Aqueous Electrolytes and Non-Electrolytes
Chapter 4 Calculation of Standard Thermodynamic Properties of Aqueous Electrolytes and Non-Electrolytes Vladimir Majer Laboratoire de Thermodynamique des Solutions et des Polymères Université Blaise Pascal Clermont II / CNRS 63177 Aubière, France Josef Sedlbauer Department of Chemistry Technical University Liberec 46117 Liberec, Czech Republic Robert H. Wood Department of Chemistry and Biochemistry University of Delaware Newark, DE 19716, USA 4.1 Introduction Thermodynamic modeling is important for understanding and predicting phase and chemical equilibria in industrial and natural aqueous systems at elevated temperatures and pressures. Such systems contain a variety of organic and inorganic solutes ranging from apolar nonelectrolytes to strong electrolytes; temperature and pressure strongly affect speciation of solutes that are encountered in molecular or ionic forms, or as ion pairs or complexes. Properties related to the Gibbs energy, such as thermodynamic equilibrium constants of hydrothermal reactions and activity coefficients of aqueous species, are required for practical use by geologists, power-cycle chemists and process engineers. Derivative properties (enthalpy, heat capacity and volume), which can be obtained from calorimetric and volumetric experiments, are useful in extrapolations when calculating the Gibbs energy at conditions remote from ambient. They also sensitively indicate evolution in molecular interactions with changing temperature and pressure. In this context, models with a sound theoretical basis are indispensable, describing with a limited number of adjustable parameters all thermodynamic functions of an aqueous system over a wide range of temperature and pressure. In thermodynamics of hydrothermal solutions, the unsymmetric standard-state convention is generally used; in this case, the standard thermodynamic properties (STP) of a solute reflect its interaction with the solvent (water), and the excess properties, related to activity coefficients, correspond to solute-solute interactions. -

Notation for States and Processes, Significance of the Word Standard in Chemical Thermodynamics, and Remarks on Commonly Tabulated Forms of Thermodynamic Functions
Pure &Appl.Chem.., Vol.54, No.6, pp.1239—1250,1982. 0033—4545/82/061239—12$03.00/0 Printed in Great Britain. Pergamon Press Ltd. ©1982IUPAC INTERNATIONAL UNION OF PURE AND APPLIED CHEMISTRY PHYSICAL CHEMISTRY DIVISION COMMISSION ON THERMODYNAMICS* NOTATION FOR STATES AND PROCESSES, SIGNIFICANCE OF THE WORD STANDARD IN CHEMICAL THERMODYNAMICS, AND REMARKS ON COMMONLY TABULATED FORMS OF THERMODYNAMIC FUNCTIONS (Recommendations 1981) (Appendix No. IV to Manual of Symbols and Terminology for Physicochemical Quantities and Units) Prepared for publication by J. D. COX, National Physical Laboratory, Teddington, UK with the assistance of a Task Group consisting of S. ANGUS, London; G. T. ARMSTRONG, Washington, DC; R. D. FREEMAN, Stiliwater, OK; M. LAFFITTE, Marseille; G. M. SCHNEIDER (Chairman), Bochum; G. SOMSEN, Amsterdam, (from Commission 1.2); and C. B. ALCOCK, Toronto; and P. W. GILLES, Lawrence, KS, (from Commission 11.3) *Membership of the Commission for 1979-81 was as follows: Chairman: M. LAFFITTE (France); Secretary: G. M. SCHNEIDER (FRG); Titular Members: S. ANGUS (UK); G. T. ARMSTRONG (USA); V. A. MEDVEDEV (USSR); Y. TAKAHASHI (Japan); I. WADSO (Sweden); W. ZIELENKIEWICZ (Poland); Associate Members: H. CHIHARA (Japan); J. F. COUNSELL (UK); M. DIAZ-PENA (Spain); P. FRANZOSINI (Italy); R. D. FREEMAN (USA); V. A. LEVITSKII (USSR); 0.SOMSEN (Netherlands); C. E. VANDERZEE (USA); National Representatives: J. PICK (Czechoslovakia); M. T. RATZSCH (GDR). NOTATION FOR STATES AND PROCESSES, SIGNIFICANCE OF THE WORD STANDARD IN CHEMICAL THERMODYNAMICS, AND REMARKS ON COMMONLY TABULATED FORMS OF THERMODYNAMIC FUNCTIONS SECTION 1. INTRODUCTION The main IUPAC Manual of symbols and terminology for physicochemical quantities and units (Ref. -
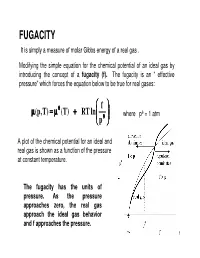
FUGACITY It Is Simply a Measure of Molar Gibbs Energy of a Real Gas
FUGACITY It is simply a measure of molar Gibbs energy of a real gas . Modifying the simple equation for the chemical potential of an ideal gas by introducing the concept of a fugacity (f). The fugacity is an “ effective pressure” which forces the equation below to be true for real gases: θθθ f µµµ ,p( T) === µµµ (T) +++ RT ln where pθ = 1 atm pθθθ A plot of the chemical potential for an ideal and real gas is shown as a function of the pressure at constant temperature. The fugacity has the units of pressure. As the pressure approaches zero, the real gas approach the ideal gas behavior and f approaches the pressure. 1 If fugacity is an “effective pressure” i.e, the pressure that gives the right value for the chemical potential of a real gas. So, the only way we can get a value for it and hence for µµµ is from the gas pressure. Thus we must find the relation between the effective pressure f and the measured pressure p. let f = φ p φ is defined as the fugacity coefficient. φφφ is the “fudge factor” that modifies the actual measured pressure to give the true chemical potential of the real gas. By introducing φ we have just put off finding f directly. Thus, now we have to find φ. Substituting for φφφ in the above equation gives: p µ=µ+(p,T)θ (T) RT ln + RT ln φ=µ (ideal gas) + RT ln φ pθ µµµ(p,T) −−− µµµ(ideal gas ) === RT ln φφφ This equation shows that the difference in chemical potential between the real and ideal gas lies in the term RT ln φφφ.φ This is the term due to molecular interaction effects. -

SAFETY DATA SHEET Halocarbon R-503
SAFETY DATA SHEET Halocarbon R-503 Section 1. Identification GHS product identifier : Halocarbon R-503 Other means of : Not available. identification Product type : Liquefied gas Product use : Synthetic/Analytical chemistry. SDS # : 007306 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : GASES UNDER PRESSURE - Liquefied gas substance or mixture HAZARDOUS TO THE OZONE LAYER - Category 1 GHS label elements Hazard pictograms : Signal word : Warning Hazard statements : Contains gas under pressure; may explode if heated. May cause frostbite. May displace oxygen and cause rapid suffocation. Harms public health and the environment by destroying ozone in the upper atmosphere. Precautionary statements General : Read and follow all Safety Data Sheets (SDS’S) before use. Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Close valve after each use and when empty. Use equipment rated for cylinder pressure. Do not open valve until connected to equipment prepared for use. Use a back flow preventative device in the piping. Use only equipment of compatible materials of construction. Always keep container in upright position. Prevention : Not applicable. Response : Not applicable. Storage : Protect from sunlight. Store in a well-ventilated place. Disposal : Refer to manufacturer or supplier for information on recovery or recycling. Hazards not otherwise : Liquid can cause burns similar to frostbite. -

Selection of Thermodynamic Methods
P & I Design Ltd Process Instrumentation Consultancy & Design 2 Reed Street, Gladstone Industrial Estate, Thornaby, TS17 7AF, United Kingdom. Tel. +44 (0) 1642 617444 Fax. +44 (0) 1642 616447 Web Site: www.pidesign.co.uk PROCESS MODELLING SELECTION OF THERMODYNAMIC METHODS by John E. Edwards [email protected] MNL031B 10/08 PAGE 1 OF 38 Process Modelling Selection of Thermodynamic Methods Contents 1.0 Introduction 2.0 Thermodynamic Fundamentals 2.1 Thermodynamic Energies 2.2 Gibbs Phase Rule 2.3 Enthalpy 2.4 Thermodynamics of Real Processes 3.0 System Phases 3.1 Single Phase Gas 3.2 Liquid Phase 3.3 Vapour liquid equilibrium 4.0 Chemical Reactions 4.1 Reaction Chemistry 4.2 Reaction Chemistry Applied 5.0 Summary Appendices I Enthalpy Calculations in CHEMCAD II Thermodynamic Model Synopsis – Vapor Liquid Equilibrium III Thermodynamic Model Selection – Application Tables IV K Model – Henry’s Law Review V Inert Gases and Infinitely Dilute Solutions VI Post Combustion Carbon Capture Thermodynamics VII Thermodynamic Guidance Note VIII Prediction of Physical Properties Figures 1 Ideal Solution Txy Diagram 2 Enthalpy Isobar 3 Thermodynamic Phases 4 van der Waals Equation of State 5 Relative Volatility in VLE Diagram 6 Azeotrope γ Value in VLE Diagram 7 VLE Diagram and Convergence Effects 8 CHEMCAD K and H Values Wizard 9 Thermodynamic Model Decision Tree 10 K Value and Enthalpy Models Selection Basis PAGE 2 OF 38 MNL 031B Issued November 2008, Prepared by J.E.Edwards of P & I Design Ltd, Teesside, UK www.pidesign.co.uk Process Modelling Selection of Thermodynamic Methods References 1. -

Questions: Physical Properties and 7 Step Process
Questions: Physical Properties and 7 Step Process 1. Why does a water-saturated sandstone typically have a higher P-wave velocity than a dry sandstone? A saturated sandstone: a. is more dense b. has a larger bulk modulus c. has a larger shear modulus d. has a higher tensile strength 2. The relative permittivity of a given rock is considered large when: a. it contains a lot of pore water b. an applied electric field results in a larger electric dipole moment c. it has a value of 30 d. b and c are correct e. a,b and c are correct 3. You measure a resistance of 16 kΩ between two parallel faces of a 2cm x 2cm x 2cm cube. Determine the resistivity. a. 320 Ωm b. 800000 Ωm c. 32000 Ωm d. 8000 Ωm 4. You are flying a gravity survey over a sedimentary basin. The flight path crosses a known dyke. What would be the expected gravity response and why? a. Gravity high over the dyke; the dyke is more dense than the background b. Gravity low over the dyke; the dyke is less dense than the background c. Gravity high over the dyke; the dyke is less dense than the background d. Gravity low over the dyke; the dyke is more dense than the background 5. You are building a road through known active Karst terrain in Ireland. Which set of physical property contrasts would be most diagnostic for locating regions where sink- holes could form? a. Karstified: low density, Limestone: high density b. Karstified: low resistivity, Limestone: high resistivity c. -

Evaluation of UNIFAC Group Interaction Parameters Usijng Properties Based on Quantum Mechanical Calculations Hansan Kim New Jersey Institute of Technology
New Jersey Institute of Technology Digital Commons @ NJIT Theses Theses and Dissertations Spring 2005 Evaluation of UNIFAC group interaction parameters usijng properties based on quantum mechanical calculations Hansan Kim New Jersey Institute of Technology Follow this and additional works at: https://digitalcommons.njit.edu/theses Part of the Chemical Engineering Commons Recommended Citation Kim, Hansan, "Evaluation of UNIFAC group interaction parameters usijng properties based on quantum mechanical calculations" (2005). Theses. 479. https://digitalcommons.njit.edu/theses/479 This Thesis is brought to you for free and open access by the Theses and Dissertations at Digital Commons @ NJIT. It has been accepted for inclusion in Theses by an authorized administrator of Digital Commons @ NJIT. For more information, please contact [email protected]. Copyright Warning & Restrictions The copyright law of the United States (Title 17, United States Code) governs the making of photocopies or other reproductions of copyrighted material. Under certain conditions specified in the law, libraries and archives are authorized to furnish a photocopy or other reproduction. One of these specified conditions is that the photocopy or reproduction is not to be “used for any purpose other than private study, scholarship, or research.” If a, user makes a request for, or later uses, a photocopy or reproduction for purposes in excess of “fair use” that user may be liable for copyright infringement, This institution reserves the right to refuse to accept a copying -

"Fluorine Compounds, Organic," In: Ullmann's Encyclopedia Of
Article No : a11_349 Fluorine Compounds, Organic GU¨ NTER SIEGEMUND, Hoechst Aktiengesellschaft, Frankfurt, Federal Republic of Germany WERNER SCHWERTFEGER, Hoechst Aktiengesellschaft, Frankfurt, Federal Republic of Germany ANDREW FEIRING, E. I. DuPont de Nemours & Co., Wilmington, Delaware, United States BRUCE SMART, E. I. DuPont de Nemours & Co., Wilmington, Delaware, United States FRED BEHR, Minnesota Mining and Manufacturing Company, St. Paul, Minnesota, United States HERWARD VOGEL, Minnesota Mining and Manufacturing Company, St. Paul, Minnesota, United States BLAINE MCKUSICK, E. I. DuPont de Nemours & Co., Wilmington, Delaware, United States 1. Introduction....................... 444 8. Fluorinated Carboxylic Acids and 2. Production Processes ................ 445 Fluorinated Alkanesulfonic Acids ...... 470 2.1. Substitution of Hydrogen............. 445 8.1. Fluorinated Carboxylic Acids ......... 470 2.2. Halogen – Fluorine Exchange ......... 446 8.1.1. Fluorinated Acetic Acids .............. 470 2.3. Synthesis from Fluorinated Synthons ... 447 8.1.2. Long-Chain Perfluorocarboxylic Acids .... 470 2.4. Addition of Hydrogen Fluoride to 8.1.3. Fluorinated Dicarboxylic Acids ......... 472 Unsaturated Bonds ................. 447 8.1.4. Tetrafluoroethylene – Perfluorovinyl Ether 2.5. Miscellaneous Methods .............. 447 Copolymers with Carboxylic Acid Groups . 472 2.6. Purification and Analysis ............. 447 8.2. Fluorinated Alkanesulfonic Acids ...... 472 3. Fluorinated Alkanes................. 448 8.2.1. Perfluoroalkanesulfonic Acids -

Thermodynamics the Study of the Transformations of Energy from One Form Into Another
Thermodynamics the study of the transformations of energy from one form into another First Law: Heat and Work are both forms of Energy. in any process, Energy can be changed from one form to another (including heat and work), but it is never created or distroyed: Conservation of Energy Second Law: Entropy is a measure of disorder; Entropy of an isolated system Increases in any spontaneous process. OR This law also predicts that the entropy of an isolated system always increases with time. Third Law: The entropy of a perfect crystal approaches zero as temperature approaches absolute zero. ©2010, 2008, 2005, 2002 by P. W. Atkins and L. L. Jones ©2010, 2008, 2005, 2002 by P. W. Atkins and L. L. Jones A Molecular Interlude: Internal Energy, U, from translation, rotation, vibration •Utranslation = 3/2 × nRT •Urotation = nRT (for linear molecules) or •Urotation = 3/2 × nRT (for nonlinear molecules) •At room temperature, the vibrational contribution is small (it is of course zero for monatomic gas at any temperature). At some high temperature, it is (3N-5)nR for linear and (3N-6)nR for nolinear molecules (N = number of atoms in the molecule. Enthalpy H = U + PV Enthalpy is a state function and at constant pressure: ∆H = ∆U + P∆V and ∆H = q At constant pressure, the change in enthalpy is equal to the heat released or absorbed by the system. Exothermic: ∆H < 0 Endothermic: ∆H > 0 Thermoneutral: ∆H = 0 Enthalpy of Physical Changes For phase transfers at constant pressure Vaporization: ∆Hvap = Hvapor – Hliquid Melting (fusion): ∆Hfus = Hliquid – -

AC Measurement System (ACMS) Option User's Manual
Physical Property Measurement System AC Measurement System (ACMS) Option User’s Manual Part Number 1084-100 C-1 Quantum Design 11578 Sorrento Valley Rd. San Diego, CA 92121-1311 USA Technical support (858) 481-4400 (800) 289-6996 Fax (858) 481-7410 Fourth edition of manual completed June 2003. Trademarks All product and company names appearing in this manual are trademarks or registered trademarks of their respective holders. U.S. Patents 4,791,788 Method for Obtaining Improved Temperature Regulation When Using Liquid Helium Cooling 4,848,093 Apparatus and Method for Regulating Temperature in a Cryogenic Test Chamber 5,311,125 Magnetic Property Characterization System Employing a Single Sensing Coil Arrangement to Measure AC Susceptibility and DC Moment of a Sample (patent licensed from Lakeshore) 5,647,228 Apparatus and Method for Regulating Temperature in Cryogenic Test Chamber 5,798,641 Torque Magnetometer Utilizing Integrated Piezoresistive Levers Foreign Patents U.K. 9713380.5 Apparatus and Method for Regulating Temperature in Cryogenic Test Chamber CONTENTS Table of Contents PREFACE Contents and Conventions ...............................................................................................................................vii P.1 Introduction .......................................................................................................................................................vii P.2 Scope of the Manual..........................................................................................................................................vii -

Lab 1 - Physical Properties of Minerals
Page - Lab 1 - Physical Properties of Minerals All rocks are composed of one or more minerals. In order to be able to identify rocks you have to be able to recognize those key minerals that make of the bulk of rocks. By definition, any substance is classified as a mineral if it meets all 5 of the following criteria: - is naturally occurring (ie. not man-made); - solid (not liquid or gaseous); - inorganic (not living and never was alive); - crystalline (has an orderly, repetitive atomic structure); - a definite chemical composition (you can write a discrete chemical formula for any mineral). Identifying an unknown mineral is like identifying any group of unknowns (leaves, flowers, bugs... etc.) You begin with a box, or a pile, of unknown minerals and try to find any group features in the samples that will allow you to separate them into smaller and smaller piles, until you are down to a single mineral and a unique name. For minerals, these group features are called physical properties. Physical properties are any features that you can use your 5 senses (see, hear, feel, taste or smell) to aid in identifying an unknown mineral. Mineral physical properties are generally organized in a mineral key and the proper use of this key will allow you to name your unknown mineral sample. The major physical properties will be discussed briefly below in the order in which they are used to identify an unknown mineral sample. Luster Luster is the way that a mineral reflects light. There are two major types of luster; metallic and non-metallic luster. -

Properties of Matter
Properties of Matter Say Thanks to the Authors Click http://www.ck12.org/saythanks (No sign in required) To access a customizable version of this book, as well as other interactive content, visit www.ck12.org CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based collaborative model termed the FlexBook®, CK-12 intends to pioneer the generation and distribution of high-quality educational content that will serve both as core text as well as provide an adaptive environment for learning, powered through the FlexBook Platform®. Copyright © 2013 CK-12 Foundation, www.ck12.org The names “CK-12” and “CK12” and associated logos and the terms “FlexBook®” and “FlexBook Platform®” (collectively “CK-12 Marks”) are trademarks and service marks of CK-12 Foundation and are protected by federal, state, and international laws. Any form of reproduction of this book in any format or medium, in whole or in sections must include the referral attribution link http://www.ck12.org/saythanks (placed in a visible location) in addition to the following terms. Except as otherwise noted, all CK-12 Content (including CK-12 Curriculum Material) is made available to Users in accordance with the Creative Commons Attribution/Non- Commercial/Share Alike 3.0 Unported (CC BY-NC-SA) License (http://creativecommons.org/licenses/by-nc-sa/3.0/), as amended and updated by Creative Commons from time to time (the “CC License”), which is incorporated herein by this reference.