Slow Lorises, Native to South and Southeast Asia, Belong to the Prosimians, an Ancient Group of Primates
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Rediscovery of Nycticebus Coucang Insularis Robinson, 1917
Sains Malaysiana 47(10)(2018): 2533–2542 http://dx.doi.org/10.17576/jsm-2018-4710-30 Rediscovery of Nycticebus coucang insularis Robinson, 1917 (Primates: Lorisidae) at Tioman Island and its Mitochondrial Genetic Assessment (Penemuan Semula Nycticebus coucang insularis Robinson, 1917 (Primate: Lorisidae) di Pulau Tioman dan Penilaian Genetik Mitokondrianya) JEFFRINE J. ROVIE-RYAN*, MILLAWATI GANI, HAN MING GAN, GILMOORE G. BOLONGON, TAN CHENG CHENG, NORAZLINDA RAZAK, NORSYAMIMI ROSLI, MOHD AZIZOL AZIZ & KALIP MATKASIM ABSTRACT Slow lorises (Nycticebus) consist of eight species native to Southeast Asia while three species are recognised in Malaysia - N. coucang, N. menagensis and N. kayan. This study reports on the rediscovery of the subspecies N. coucang insularis Robinson, 1917 in Tioman Island and the genetic assessment of its mitochondrial DNA variation. Morphological measurements conform the specimen as the putative N. coucang but with distinct colour and markings. Two mitochondrial DNA segments (cytochrome b and control region) were produced from the subspecies representing their first registered sequences in GenBank. Genetically, the subspecies showed 99% of nucleotide similarity to N. coucang species type for both the DNA segments and constitute its own unique haplotype. Phylogenetic trees constructed using three methods (neighbour joining, maximum likelihood and Bayesian inference) showed two major groups within Nycticebus; the basal group was formed by N. pygmaeus while the second group consisted of the remaining Nycticebus species. The phylogenetic position of the subspecies, however, remains unresolved due to the observed mixing between N. coucang and N. bengalensis. Several reasons could lead to this condition including the lack of well documented voucher specimens and the short DNA fragments used. -

Molecular Data Confirm the Presence of Nycticebus Bengalensis on Langkawi Island, Malaysia
BIODIVERSITAS ISSN: 1412-033X Volume 20, Number 4, April 2019 E-ISSN: 2085-4722 Pages: 1115-1120 DOI: 10.13057/biodiv/d200424 Molecular data confirm the presence of Nycticebus bengalensis on Langkawi Island, Malaysia BADRUL MUNIR MD-ZAIN1,, KHAIRUL SYA’ADAH MOHHOYUA1, NOR RAHMAN AIFAT1, EHWAN NGADI1, NORSHAQINAH AYOB1, JEFFRINE JAPNING ROVIE-RYAN2, AHMAD AMPENG3, ABD RAHMAN MOHD-RIDWAN1,4, MARY E BLAIR5, MUHAMMAD ABU BAKAR ABDUL-LATIFF6 1School of Environmental and Natural Resource Sciences, Faculty of Science and Technology, Universiti Kebangsaan Malaysia. 43600, Bangi, Selangor, Malaysia. Tel.: +60-389-213200, email: [email protected], [email protected] 2Department of Wildlife and National Park (DWNP) Peninsular Malaysia, Km 10 Jalan Cheras, 56100 Kuala Lumpur, Malaysia 3Sarawak Forest Department, Wisma Sumber Alam Jalan Stadium, 93660 Petra Jaya, Kuching, Sarawak, Malaysia 4Centre for Pre-University Studies, Universiti Malaysia Sarawak,94300 Kota Samarahan, Sarawak, Malaysia 5Center for Biodiversity and Conservation, American Museum of Natural History, Central Park West & 79th St, New York, NY 10024, United States 6Centre of Research for Sustainable Uses of Natural Resources (CoR-SUNR), Faculty of Applied Sciences and Technology, Universiti Tun Hussein Onn Malaysia (Pagoh Campus). 84000, Muar, Johor, Malaysia Manuscript received: 23 February 2019. Revision accepted: 24 March 2019. Abstract. Md-Zain BM, Mohhoyua KS, Aifat NR, Ngadi E, Ayob N, Rovie-Ryan JJ, Ampeng A, Mohd-Ridwan AR, Blair ME, Abdul-Latiff MAB. 2019. Molecular data confirm the presence of Nycticebus bengalensis on Langkawi Island, Malaysia. Biodiversitas 20: 1115- 1120. Recent taxonomic reviews have stated the possibility of Bengal Slow Loris (Nycticebus bengalensis) presence in the Northern part of the Malay Peninsula. -

Keshav Ravi by Keshav Ravi
by Keshav Ravi by Keshav Ravi Preface About the Author In the whole world, there are more than 30,000 species Keshav Ravi is a caring and compassionate third grader threatened with extinction today. One prominent way to who has been fascinated by nature throughout his raise awareness as to the plight of these animals is, of childhood. Keshav is a prolific reader and writer of course, education. nonfiction and is always eager to share what he has learned with others. I have always been interested in wildlife, from extinct dinosaurs to the lemurs of Madagascar. At my ninth Outside of his family, Keshav is thrilled to have birthday, one personal writing project I had going was on the support of invested animal advocates, such as endangered wildlife, and I had chosen to focus on India, Carole Hyde and Leonor Delgado, at the Palo Alto the country where I had spent a few summers, away from Humane Society. my home in California. Keshav also wishes to thank Ernest P. Walker’s Just as I began to explore the International Union for encyclopedia (Walker et al. 1975) Mammals of the World Conservation of Nature (IUCN) Red List species for for inspiration and the many Indian wildlife scientists India, I realized quickly that the severity of threat to a and photographers whose efforts have made this variety of species was immense. It was humbling to then work possible. realize that I would have to narrow my focus further down to a subset of species—and that brought me to this book on the Endangered Mammals of India. -

Pongo Abelii)
UvA-DARE (Digital Academic Repository) Orangutan diet: lessons from and for the wild Hardus, M.E. Publication date 2012 Link to publication Citation for published version (APA): Hardus, M. E. (2012). Orangutan diet: lessons from and for the wild. General rights It is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), other than for strictly personal, individual use, unless the work is under an open content license (like Creative Commons). Disclaimer/Complaints regulations If you believe that digital publication of certain material infringes any of your rights or (privacy) interests, please let the Library know, stating your reasons. In case of a legitimate complaint, the Library will make the material inaccessible and/or remove it from the website. Please Ask the Library: https://uba.uva.nl/en/contact, or a letter to: Library of the University of Amsterdam, Secretariat, Singel 425, 1012 WP Amsterdam, The Netherlands. You will be contacted as soon as possible. UvA-DARE is a service provided by the library of the University of Amsterdam (https://dare.uva.nl) Download date:29 Sep 2021 4 Behavioral, Ecological, and Evolutionary Aspects of Meat-Eating by Sumatran Orangutans (Pongo abelii) Madeleine E. Hardus, Adriano R. Lameira, Astri Zulfa, S. Suci Utami Atmoko, Han de Vries, Serge A. Wich Abstract Meat-eating is an important aspect of human evolution, but how meat became a substantial component of the human diet is still poorly understood. Meat eating in our closest relatives, the great apes, may provide insight into the emergence of this trait, but most existing data are for chimpanzees. -

Downloaded from Brill.Com09/27/2021 09:14:05PM Via Free Access 218 Rode-Margono & Nekaris – Impact of Climate and Moonlight on Javan Slow Lorises
Contributions to Zoology, 83 (4) 217-225 (2014) Impact of climate and moonlight on a venomous mammal, the Javan slow loris (Nycticebus javanicus Geoffroy, 1812) Eva Johanna Rode-Margono1, K. Anne-Isola Nekaris1, 2 1 Oxford Brookes University, Gipsy Lane, Headington, Oxford OX3 0BP, UK 2 E-mail: [email protected] Keywords: activity, environmental factors, humidity, lunarphobia, moon, predation, temperature Abstract Introduction Predation pressure, food availability, and activity may be af- To secure maintenance, survival and reproduction, fected by level of moonlight and climatic conditions. While many animals adapt their behaviour to various factors, such nocturnal mammals reduce activity at high lunar illumination to avoid predators (lunarphobia), most visually-oriented nocturnal as climate, availability of resources, competition, preda- primates and birds increase activity in bright nights (lunarphilia) tion, luminosity, habitat fragmentation, and anthropo- to improve foraging efficiency. Similarly, weather conditions may genic disturbance (Kappeler and Erkert, 2003; Beier influence activity level and foraging ability. We examined the 2006; Donati and Borgognini-Tarli, 2006). According response of Javan slow lorises (Nycticebus javanicus Geoffroy, to optimal foraging theory, animal behaviour can be seen 1812) to moonlight and temperature. We radio-tracked 12 animals as a trade-off between the risk of being preyed upon in West Java, Indonesia, over 1.5 years, resulting in over 600 hours direct observations. We collected behavioural and environmen- and the fitness gained from foraging (Charnov, 1976). tal data including lunar illumination, number of human observ- Perceived predation risk assessed through indirect cues ers, and climatic factors, and 185 camera trap nights on potential that correlate with the probability of encountering a predators. -

Slow Loris Finds Itself Stranded April 30Th 2016
Slow loris finds itself stranded far away from home... at Yishun carpark PHOTO: YouTube screengrabs ASIAONE Apr 30, 2016 SINGAPORE - It has a permanent look of surprise on its face, but this slow loris was probably really afraid when it found itself surrounded by a concrete jungle instead of the lush greenery she is used to. Earlier this month, officers from the Animal Concerns Research & Education Society (Acres) were notified of a slow loris stranded in a multi-storey carpark at Yishun Central. The resident who found the nocturnal animal recognised it immediately and knew that it was not in a place it belonged. One of Singapore's critically endangered creatures, slow lorises are usually found deep in the nature reserves of Singapore where they enjoy a diet of fruit, sap, nectar, bird eggs and insects. In a video uploaded on Acres' YouTube account, the slow loris can be seen perched on the ledge three storeys above ground. With thick gloves to protect himself from the the animal's strong and toxic bite, an Acres officer grabs hold of it and brings it carefully to safety. Manager of Acres' wildlife department, Kalai, told AsiaOne in a phone interview that the Sunda slow loris, which is native to Singapore, is usually not found near residential areas. So how exactly did this "young adult" female slow loris get to a carpark in Yishun Central? Kalai says there are just two possible scenarios. One possibility was that it had been sold as part of the illegal pet trade and escaped from captivity, while the other possibility was that it could have accidentally 'hitched' a ride out of the nature reserve on the car of an unsuspecting visitor. -

In Situ Conservation
NEWSN°17/DECEMBER 2020 Editorial IN SITU CONSERVATION One effect from 2020 is for sure: Uncertainty. Forward planning is largely News from the Little Fireface First, our annual SLOW event was impossible. We are acting and reacting Project, Java, Indonesia celebrated world-wide, including along the current situation caused by the By Prof K.A.I. Nekaris, MA, PhD by project partners Kukang Rescue Covid-19 pandemic. All zoos are struggling Director of the Little Fireface Project Program Sumatra, EAST Vietnam, Love economically after (and still ongoing) Wildlife Thailand, NE India Primate temporary closures and restricted business. The Little Fireface Project team has Investments in development are postponed Centre India, and the Bangladesh Slow at least. Each budget must be reviewed. been busy! Despite COVID we have Loris Project, to name a few. The end In the last newsletter we mentioned not been able to keep up with our wild of the week resulted in a loris virtual to forget about the support of the in situ radio collared slow lorises, including conference, featuring speakers from conservation efforts. Some of these under welcoming many new babies into the the helm of the Prosimian TAG are crucial 11 loris range countries. Over 200 for the survival of species – and for a more family. The ‘cover photo’ you see here people registered, and via Facebook sustainable life for the people involved in is Smol – the daughter of Lupak – and Live, more than 6000 people watched rd some of the poorest countries in the world. is our first 3 generation birth! Having the event. -
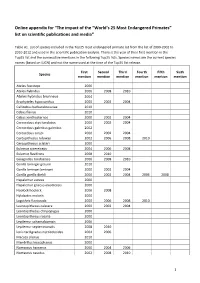
Online Appendix for “The Impact of the “World's 25 Most Endangered
Online appendix for “The impact of the “World’s 25 Most Endangered Primates” list on scientific publications and media” Table A1. List of species included in the Top25 most endangered primate list from the list of 2000-2002 to 2010-2012 and used in the scientific publication analysis. There is the year of their first mention in the Top25 list and the consecutive mentions in the following Top25 lists. Species names are the current species names (based on IUCN) and not the name used at the time of the Top25 list release. First Second Third Fourth Fifth Sixth Species mention mention mention mention mention mention Ateles fusciceps 2006 Ateles hybridus 2006 2008 2010 Ateles hybridus brunneus 2004 Brachyteles hypoxanthus 2000 2002 2004 Callicebus barbarabrownae 2010 Cebus flavius 2010 Cebus xanthosternos 2000 2002 2004 Cercocebus atys lunulatus 2000 2002 2004 Cercocebus galeritus galeritus 2002 Cercocebus sanjei 2000 2002 2004 Cercopithecus roloway 2002 2006 2008 2010 Cercopithecus sclateri 2000 Eulemur cinereiceps 2004 2006 2008 Eulemur flavifrons 2008 2010 Galagoides rondoensis 2006 2008 2010 Gorilla beringei graueri 2010 Gorilla beringei beringei 2000 2002 2004 Gorilla gorilla diehli 2000 2002 2004 2006 2008 Hapalemur aureus 2000 Hapalemur griseus alaotrensis 2000 Hoolock hoolock 2006 2008 Hylobates moloch 2000 Lagothrix flavicauda 2000 2006 2008 2010 Leontopithecus caissara 2000 2002 2004 Leontopithecus chrysopygus 2000 Leontopithecus rosalia 2000 Lepilemur sahamalazensis 2006 Lepilemur septentrionalis 2008 2010 Loris tardigradus nycticeboides -

Slow Loris Forest Protector Teacher's Pack
2 www.nocturama.org https://www.facebook.com/pages/Little-Fireface-Project/ littlefi[email protected] INTRODUCTION Welcome! Welcome to the Slow Loris—Forest Protector’s Teacher’s Pack and Learning Exercise Book. The purpose of this short booklet is to explain how to use the book and to help to reinforce its message through a series of fun and easy-to- We at the Little Fireface Project (LFP) our so glad you are using this teacher’s use exercises. pack. First we want to tell you about ourselves and our passion about one of the world’s most unique little primates. LFP is a UK Charity based out of Ox- ford Brookes University set up to help save the slow loris through studying In this pack, you will find the following materials to help you and your students them in the wild and through education projects. explore the story of two night-active (nocturnal) primates, slow lorises: a moth- er (Tereh—speedy) and her young son (Bunga—flower). Tereh lovingly teaches Why the slow loris and why a charity dedicated to one group of animals? Well, her son the life skills he needs to be a grown-up slow loris. At the same time, the eight species of slow lorises, found only in Asia, are facing a tough time. Bunga learns that by doing his job in the forest, he helps the forest to grow, They are threatened for many reasons beyond the habitat loss causing many of while helping protect the crops grown by people. Asia’s species to go extinct. -

Science Journals
SCIENCE ADVANCES | REVIEW PRIMATOLOGY 2017 © The Authors, some rights reserved; Impending extinction crisis of the world’s primates: exclusive licensee American Association Why primates matter for the Advancement of Science. Distributed 1 2 3 4 under a Creative Alejandro Estrada, * Paul A. Garber, * Anthony B. Rylands, Christian Roos, Commons Attribution 5 6 7 7 Eduardo Fernandez-Duque, Anthony Di Fiore, K. Anne-Isola Nekaris, Vincent Nijman, NonCommercial 8 9 10 10 Eckhard W. Heymann, Joanna E. Lambert, Francesco Rovero, Claudia Barelli, License 4.0 (CC BY-NC). Joanna M. Setchell,11 Thomas R. Gillespie,12 Russell A. Mittermeier,3 Luis Verde Arregoitia,13 Miguel de Guinea,7 Sidney Gouveia,14 Ricardo Dobrovolski,15 Sam Shanee,16,17 Noga Shanee,16,17 Sarah A. Boyle,18 Agustin Fuentes,19 Katherine C. MacKinnon,20 Katherine R. Amato,21 Andreas L. S. Meyer,22 Serge Wich,23,24 Robert W. Sussman,25 Ruliang Pan,26 27 28 Inza Kone, Baoguo Li Downloaded from Nonhuman primates, our closest biological relatives, play important roles in the livelihoods, cultures, and religions of many societies and offer unique insights into human evolution, biology, behavior, and the threat of emerging diseases. They are an essential component of tropical biodiversity, contributing to forest regeneration and ecosystem health. Current information shows the existence of 504 species in 79 genera distributed in the Neotropics, mainland Africa, Madagascar, and Asia. Alarmingly, ~60% of primate species are now threatened with extinction and ~75% have de- clining populations. This situation is the result of escalating anthropogenic pressures on primates and their habitats— mainly global and local market demands, leading to extensive habitat loss through the expansion of industrial agri- http://advances.sciencemag.org/ culture, large-scale cattle ranching, logging, oil and gas drilling, mining, dam building, and the construction of new road networks in primate range regions. -

World's Most Endangered Primates
Primates in Peril The World’s 25 Most Endangered Primates 2016–2018 Edited by Christoph Schwitzer, Russell A. Mittermeier, Anthony B. Rylands, Federica Chiozza, Elizabeth A. Williamson, Elizabeth J. Macfie, Janette Wallis and Alison Cotton Illustrations by Stephen D. Nash IUCN SSC Primate Specialist Group (PSG) International Primatological Society (IPS) Conservation International (CI) Bristol Zoological Society (BZS) Published by: IUCN SSC Primate Specialist Group (PSG), International Primatological Society (IPS), Conservation International (CI), Bristol Zoological Society (BZS) Copyright: ©2017 Conservation International All rights reserved. No part of this report may be reproduced in any form or by any means without permission in writing from the publisher. Inquiries to the publisher should be directed to the following address: Russell A. Mittermeier, Chair, IUCN SSC Primate Specialist Group, Conservation International, 2011 Crystal Drive, Suite 500, Arlington, VA 22202, USA. Citation (report): Schwitzer, C., Mittermeier, R.A., Rylands, A.B., Chiozza, F., Williamson, E.A., Macfie, E.J., Wallis, J. and Cotton, A. (eds.). 2017. Primates in Peril: The World’s 25 Most Endangered Primates 2016–2018. IUCN SSC Primate Specialist Group (PSG), International Primatological Society (IPS), Conservation International (CI), and Bristol Zoological Society, Arlington, VA. 99 pp. Citation (species): Salmona, J., Patel, E.R., Chikhi, L. and Banks, M.A. 2017. Propithecus perrieri (Lavauden, 1931). In: C. Schwitzer, R.A. Mittermeier, A.B. Rylands, F. Chiozza, E.A. Williamson, E.J. Macfie, J. Wallis and A. Cotton (eds.), Primates in Peril: The World’s 25 Most Endangered Primates 2016–2018, pp. 40-43. IUCN SSC Primate Specialist Group (PSG), International Primatological Society (IPS), Conservation International (CI), and Bristol Zoological Society, Arlington, VA. -

July 2018 Vol.6 No.1 Journal of Indonesian Natural History Editors Dr
Journal of Indonesian Natural History July 2018 Vol.6 No.1 Journal of Indonesian Natural History Editors Dr. Wilson Novarino Dr. Carl Traeholt Associate Professor for Biology Programme Director, Southeast Asia Department of Biology Research and Conservation Division Andalas University, Indonesia Copenhagen Zoo, Denmark Email: [email protected] Email: [email protected] Editorial board Dr. Ardinis Arbain Dr. Ramadhanil Pitopang University of Andalas, Indonesia Tadulako University, Indonesia Indra Arinal Dr. Lilik Budi Prasetyo National Park Management, Department of Forestry Indonesia Bogor Institute of Agriculture, Indonesia Dr. Ahimsa Campos-Arceiz Dr. Dewi Malia Prawiradilaga Nottingham University Malaysia Campus, Malaysia Indonesia Institute of Science, Indonesia Dr. Mads Frost Bertelsen Dr. Rizaldi Research and Conservation Division, Copenhagen Zoo, Denmark University of Andalas, Indonesia Dr. Susan Cheyne Dr. Dewi Imelda Roesma Oxford University, Wildlife Research Unit, United Kingdom University of Andalas, Indonesia Bjorn Dahlen Dr. Jeffrine Rovie Ryan Green Harvest Environmental Sdn. Bhd, Malaysia Wildlife Forensics Lab, Dept. of Wildlife and National Parks, Malaysia Dr. Niel Furey Boyd Simpson Centre for Biodiversity Conservation, Royal University of Phnom Penh, Cambodia Research and Conservation Division, Copenhagen Zoo, Denmark Dr. Benoit Goossens Robert B. Stuebing Cardiff University, United Kingdom Herpetology and Conservation Biology, Indonesia Dr. Djoko Iskandar Dr. Sunarto Bandung Institute of Technology, Indonesia WWF-Indonesia