Survey of Haloacetic Acids in Household Drinking Water Produced from Thermal Desalination in Kuwait H.F
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

كتاب-الاحصاء-السنوي-الكهرباء-Compressed.Pdf
;jÁÊ“’\;Ï’ÂÄ State Of Kuwait ;ÍÊfiâ ’\;Ô]ë t¸\;g ]i— 2 0 2 0 ;ÎÄÅq i∏\;ÏÕ] ’\Â;Ô]∏\Â;Ô]dÖ‚“ ’\;ÎÑ\Ü ;ÄŬ’\;C;ÏË]dÖ‚“ ’\;ÏÕ] ’\;D 4 4 ” ; W ^ اﻟﻄﺎﻗﺔ اﻟﻜﻬﺮﺑﺎﺋﻴﺔ Electrical Energy Electricity & W ater & Renew able Energy f ;ÍÊfiâ’\;Ô]ët¸\;g]i— Statistical Year Book ;k]ŸÊ÷¬∏\;á—ÖŸÂ;Ô]ët¸\;ÎÑ\Äb;U;ÉË fihÂ;Ä\Å¡b M instry O 2021;U;Ñ\Åêb Statistical Year 2020 ( Electrical Energy ) Edition 44 “A” ;€ËtÖ’\;˛fl∂Ö’\;˛!\;€âd ;ÿ˛Ü]fi˛Ÿ;˛‰ˇÑ˛ÅÕ˛Â;\˛ÑÊ›;ˇÖ˛⁄˛Œ˛’\Â;˛Ô]˲î;ä˛⁄ç’\;◊˛¬˛p;Ͳɒ\;Ê˛·;Dˇ ’˛b;”˛’Ç;ٰ˛!\;ˇœ˛÷˛|;]˛Ÿ;˛ۚ;g]˛â˛¢\Â;˛Ø˛fiâ’\;Ä˛Å˛¡;\Ê˛⁄ˇ÷˛¬iˆ; C;;‡Ê˛⁄ˇ÷˛¬Á;˛‹ÊŒ˛’;k]Á˛˙\;◊ˇë ˛Á;ˇۚ;œ¢]˛d; ;C5D;ÏÁ˙\;U;ä›ÊÁ;ÎÑÊà ;ÓÅ ∏\;Ęe’\;3Ÿ^;Ê⁄â’\;ft]ê;ÎÖït @Åbjó€a@ãibßa@áº˛a@“aÏ„@ÑÓì€a ;jÁÊ“’\;Ï’ÂÄ;3Ÿ^ H.H Sheikh Nawaf Al-Ahmed Al-Jaber Al-Sabah The Amir of the State of Kuwait @Åbjó€a@ãibßa@áº˛a@›»ìfl@ÑÓì€a@Ï8 ;jÁÊ“’\;Ï’ÂÄ;Å‚¡;È’Â H.H Sheikh Mishal Al-Ahmed Al-Jaber Al-Sabah The Crown Prince of the State of Kuwait تقديم تعمل وزارة الكهرباء واملاء جاهدةً على املشاركة يف حتقيق رؤية 2035 التنموية يف جمال توليد الطاقة الكهربائية وحتلية املياه ، ومن اجلهود الواضحة يف هذا اجملال إدخال تكنولوجيا الطاقة البديلة )املتجددة( تدرجيياً للعمل جنباً إىل جنب مع مصادر الطاقة اﻷخرى . إن اهلدف املخطط له من قِبل الوزارة ضمن رؤية 2035 هو الوصول بإنتاج الطاقة الكهربائيةة البديلة إىل ما نسبته 15% من حاجة البﻻد الكلية من الطاقة الكهربائية وحتقيق اﻷمن املائي ، وذلك من خﻻل حتفيز برنامج الشراكة بني القطاعني العام واخلاص يف تنفيذ بعض مشاريع الطاقة الكهربائية وحتلية املياه . -

PRIVATE HOUSING MURSHID 2014 Brief on Real Estate Union
PRIVATE HOUSING MURSHID 2014 Brief on Real Estate Union Real Estate Association was established in 1990 by a distinguished group headed by late Sheikh Nasser Saud Al-Sabah who exerted a lot of efforts to establish the Association. Bright visionary objectives were the motives to establish the Association. The Association works to sustainably fulfil these objectives through institutional mechanisms, which provide the essential guidelines and controls. The Association seeks to act as an umbrella gathering the real estate owners and represent their common interests in the business community, overseeing the rights of the real estate professionals and further playing a prominent role in developing the real estate sector to be a major and influential player in the economic decision-making in Kuwait. The Association also offers advisory services that improve the real estate market in Kuwait and enhance the safety of the real estate investments, which result in increasing the market attractiveness for more investment. The Association considers as a priority keeping the investment interests of its members and increase the membership base to include all owners segments of the commercial and investment real estate. Summary Private housing segment is the single most important segment of real estate industry in Kuwait. The land parcels zoned for private housing account for 78% of all land parcels in Kuwait and the segment is the main source of wealth (as family houses) for most of the households. This report is written with an objective to provide a comprehensive analysis of this segment with the historical trends of prices, rental activities, and impact of land features on property prices. -

Food and Beverages Logo Name Address
Food And Beverages Logo Name Address Salmiya - Gulf Road , Tel: Cucina - 25% discount 25770000 Salmiya - Gulf Road , Tel: Chococafe- 25% discount 25770000 Salmiya - Gulf Road , Tel: Symphony Gourmet - 25% discount 25770000 Salmiya - Gulf Road , Tel: Luna - 25% discount 25770000 Al Bida Road, Al -Ta'awn Al Bustan - 25% discount Street, Salwa Tel: 25673410 Al Bida Road, Al -Ta'awn Eedam - 25% discount on homemade items only Street, Salwa Tel: 25673420 Al Bida Road, Al -Ta'awn Al Boom - 25% discount Street, Salwa Tel: 25673430 Al Bida Road, Al -Ta'awn Peacock - 25% discount during lunch only Street, Salwa Tel: 25673440 Marina mall / liwan/ the Nestle Toll House café - 15% discount village / Levels Tel : 22493773 Sharq, Al Hamra luxury Sushi bar - 12% discount center, 1st floor Sharq, Al Hamra luxury Versus Varsace Café - 12% discount center, 1st floor The Village, Kipco Tower Upper Crust - 10% discount Sharq Tel : 1821872 Murooj Sabhan Tel : Crumbs - 10% discount 22050270 Dar Al Awadhi, Kuwait Munch - 10% discount City Tel : 22322747 Gulf Road, Across from Mais Al Ghanim - 10% discount Kuwait Towers, Spoons Complex Tel: 22250655 Bneid Al gar - Al bastaki Mawal Lebnan Café & Restaurant - 15% discount hotel - 14th floor Tel : 22575169 Miral complex Tel : Debs Al reman - 15% Discount 22493773 Avenues Mall Tel : Boccini Pizzeria - 15% discount 22493773 Salmiya, Salem Mubarak ibis Hotel Restaurants- 20% discount Street and Sharq Tel : 25713872 Salmiya, Tel: 25742090- Dag Ayoush- 15% discount 55407179 Salmiya salem almubarak street - Basbakan- -

Semantic Innovation and Change in Kuwaiti Arabic: a Study of the Polysemy of Verbs
` Semantic Innovation and Change in Kuwaiti Arabic: A Study of the Polysemy of Verbs Yousuf B. AlBader Thesis submitted to the University of Sheffield in fulfilment of the requirements for the degree of Doctor of Philosophy in the School of English Literature, Language and Linguistics April 2015 ABSTRACT This thesis is a socio-historical study of semantic innovation and change of a contemporary dialect spoken in north-eastern Arabia known as Kuwaiti Arabic. I analyse the structure of polysemy of verbs and their uses by native speakers in Kuwait City. I particularly report on qualitative and ethnographic analyses of four motion verbs: dašš ‘enter’, xalla ‘leave’, miša ‘walk’, and i a ‘run’, with the aim of establishing whether and to what extent linguistic and social factors condition and constrain the emergence and development of new senses. The overarching research question is: How do we account for the patterns of polysemy of verbs in Kuwaiti Arabic? Local social gatherings generate more evidence of semantic innovation and change with respect to the key verbs than other kinds of contexts. The results of the semantic analysis indicate that meaning is both contextually and collocationally bound and that a verb’s meaning is activated in different contexts. In order to uncover the more local social meanings of this change, I also report that the use of innovative or well-attested senses relates to the community of practice of the speakers. The qualitative and ethnographic analyses demonstrate a number of differences between friendship communities of practice and familial communities of practice. The groups of people in these communities of practice can be distinguished in terms of their habits of speech, which are conditioned by the situation of use. -

Al Koot Kuwait Provider Network
AlKoot Insurance & Reinsurance Partner Contact Details: Kuwait network providers list Partner name: Globemed Tel: +961 1 518 100 Email: [email protected] Agreement type Provider Name Provider Type Provider Address City Country Partner Al Salam International Hospital Hospital Bnaid Al Gar Kuwait City Kuwait Partner London Hospital Hospital Al Fintas Kuwait City Kuwait Partner Dar Al Shifa Hospital Hospital Hawally Kuwait City Kuwait Partner Al Hadi Hospital Hospital Jabriya Kuwait City Kuwait Partner Al Orf Hospital Hospital Al Jahra Kuwait City Kuwait Partner Royale Hayat Hospital Hospital Jabriyah Kuwait City Kuwait Partner Alia International Hospital Hospital Mahboula Kuwait City Kuwait Partner Sidra Hospital Hospital Al Reggai Kuwait City Kuwait Partner Al Rashid Hospital Hospital Salmiya Kuwait City Kuwait Partner Al Seef Hospital Hospital Salmiya Kuwait City Kuwait Partner New Mowasat Hospital Hospital Salmiya Kuwait City Kuwait Partner Taiba Hospital Hospital Sabah Al-Salem Kuwait City Kuwait Partner Kuwait Hospital Hospital Sabah Al-Salem Kuwait City Kuwait Partner Medical One Polyclinic Medical Center Al Da'iyah Kuwait City Kuwait Partner Noor Clinic Medical Center Al Ageila Kuwait City Kuwait Partner Quttainah Medical Center Medical Center Al Shaab Al Bahri Kuwait City Kuwait Partner Shaab Medical Center Medical Center Al Shaab Al Bahri Kuwait City Kuwait Partner Al Saleh Clinic Medical Center Abraq Kheetan Kuwait City Kuwait Partner Global Medical Center Medical Center Benaid Al qar Kuwait City Kuwait Partner New Life -

NBK Annual Report 2020 3 1 Strategic Review
Maintaining Course Annual Report 2020 Maintaining Course HH Sheikh HH Sheikh Nawaf Al-Ahmad Al-Jaber Al-Sabah Mishal Al-Ahmad Al-Jaber Al-Sabah Emir of the State of Kuwait Crown Prince of the State of Kuwait At a glance 97,996 57,722 4 96,524 54,584 Continents 51,124 90,447 7,179 2018 2019 2020 2018 2019 2020 Global Employees Total Assets (USD million) Loans, Advances and Islamic Financing (USD million) 56,403 1.72% 10,575 10,463 52,533 NPL Ratio 9,724 47,449 7.0% Return on Average 2018 2019 2020 2018 2019 2020 Equity Total Equity (USD million) Customer Deposits (USD million) 1,323 2,953 98.0 1,222 2,913 USD Billion Total 812 2,778 Assets 18.4% 2018 2019 2020 2018 2019 2020 Capital Adequacy Ratio Net Profit Attributable Net Operating Income (USD million) to Shareholders (USD million) About NBK Founded in Kuwait City in 1952, the Ownership 1. Consumer and private banking National Bank of Kuwait S.A.K.P. (‘NBK’ 2. Corporate banking or the ‘Bank’) is the country’s longest- Established by a group of leading Kuwaiti 3. Islamic banking (through subsidiary serving local bank – and the first merchants, NBK has retained its core Boubyan Bank) shareholding company in Kuwait and the shareholder base since inception. Its 4. Investment banking and asset Gulf Cooperation Council (‘GCC’). Over shares have been listed on the Kuwait management (through subsidiary NBK 68 years, NBK has established itself as Stock Exchange since 1984, with a Capital) Kuwait’s leading financial institution, single shareholder (the Public Institution extending its franchise across the Middle for Social Security) owning more than East and beyond, to global markets. -
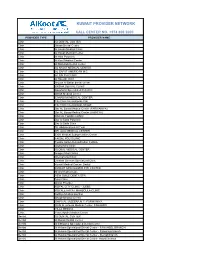
Kuwait Provider Network
KUWAIT PROVIDER NETWORK CALL CENTER NO. +974 800 2000 PROVIDER TYPE PROVIDER NAME Clinic 32 DENTAL CENTER Clinic Aknan Dental Center Clinic Al Ansari Medical Clinic Clinic Al Hayat Medical Center Clinic Al Hilal Polyclinic Clinic Al Kout Medical Center Clinic Al Muhallab Dental Center Clinic AL NAJAT MEDICAL CENTER Clinic AL SAFAT AMERICAN M.C. Clinic AL SALEH CLINIC Clinic Al Sayegh Clinic Clinic Arouss Al Bahar dental center Clinic BASMA DENTAL CLINIC Clinic Boushahri Specialized Polyclinic Clinic British Medical Center Clinic CANADIAN MEDICAL CENTER Clinic City Clinic International- Fah Clinic City Clinic International- Mirgab Clinic Dar AL Baraa Medical Center (FARWANIYA) Clinic Dar AL Baraa Medical Center (JABRIYA) Clinic DAR AL FOUAD CLINIC Clinic Dar Al Saha Polyclinic Clinic Dar Al Shifa Clinic Clinic Dr. Abdulmohsen Al Terki Clinic DR. ALIA MEDICAL CENTER Clinic EXIR Medical Subspecialties Center Clinic FAISAL POLYCLINIC Clinic Fawzia Sultan Rehabilitation Institute Clinic Global Med Clinic Clinic GLOBAL MEDICAL CENTER Clinic Images Med Clinics Clinic International Clinic Clinic Jarallah German Specialized Clinic Clinic Kuwait Medical Center- Dental Clinic KUWAIT SPECIALIZED EYE CENTER Clinic Metro Medical Care Clinic NEW SMILE DENTA SPA Clinic Noor Clinic Clinic Oman Provider Clinic ROYAL CITY CLINIC - JLEEB Clinic ROYALE HAYAT MAHBOULA CLINIC Clinic Salhiya Medical pavilion Clinic Shaab Medical Center Clinic SHIFA AL JAZEERA M.C.-FARWANIYA Clinic Shifa Al Jazeera Medical Center, FAHAHEEL Clinic VILLA MEDICA Clinic Yiaco Apollo -

Comparative Geomatic Analysis of Historic Development, Trends, And
University of Arkansas, Fayetteville ScholarWorks@UARK Theses and Dissertations 5-2015 Comparative Geomatic Analysis of Historic Development, Trends, and Functions of Green Space in Kuwait City From 1982-2014 Yousif Abdullah University of Arkansas, Fayetteville Follow this and additional works at: http://scholarworks.uark.edu/etd Part of the Near and Middle Eastern Studies Commons, Physical and Environmental Geography Commons, and the Urban Studies and Planning Commons Recommended Citation Abdullah, Yousif, "Comparative Geomatic Analysis of Historic Development, Trends, and Functions of Green Space in Kuwait City From 1982-2014" (2015). Theses and Dissertations. 1116. http://scholarworks.uark.edu/etd/1116 This Thesis is brought to you for free and open access by ScholarWorks@UARK. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of ScholarWorks@UARK. For more information, please contact [email protected], [email protected]. Comparative Geomatic Analysis of Historic Development, Trends, and Functions Of Green Space in Kuwait City From 1982-2014. Comparative Geomatic Analysis of Historic Development, Trends, and Functions Of Green Space in Kuwait City From 1982-2014. A Thesis submitted in partial fulfillment Of the requirements for the Degree of Master of Art in Geography By Yousif Abdullah Kuwait University Bachelor of art in GIS/Geography, 2011 Kuwait University Master of art in Geography May 2015 University of Arkansas This thesis is approved for recommendation to the Graduate Council. ____________________________ Dr. Ralph K. Davis Chair ____________________________ ___________________________ Dr. Thomas R. Paradise Dr. Fiona M. Davidson Thesis Advisor Committee Member ____________________________ ___________________________ Dr. Mohamed Aly Dr. Carl Smith Committee Member Committee Member ABSTRACT This research assessed green space morphology in Kuwait City, explaining its evolution from 1982 to 2014, through the use of geo-informatics, including remote sensing, geographic information systems (GIS), and cartography. -
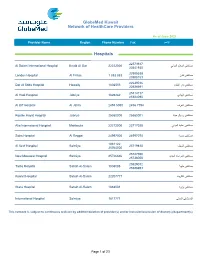
Globemed Kuwait Network of Providers Exc KSEC and BAYAN
GlobeMed Kuwait Network of HealthCare Providers As of June 2021 Phone ﺍﻻﺳﻡ ProviderProvider Name Name Region Region Phone Number FFaxax Number Number Hospitals 22573617 ﻣﺳﺗﺷﻔﻰ ﺍﻟﺳﻼﻡ ﺍﻟﺩﻭﻟﻲ Al Salam International Hospital Bnaid Al Gar 22232000 22541930 23905538 ﻣﺳﺗﺷﻔﻰ ﻟﻧﺩﻥ London Hospital Al Fintas 1 883 883 23900153 22639016 ﻣﺳﺗﺷﻔﻰ ﺩﺍﺭ ﺍﻟﺷﻔﺎء Dar Al Shifa Hospital Hawally 1802555 22626691 25314717 ﻣﺳﺗﺷﻔﻰ ﺍﻟﻬﺎﺩﻱ Al Hadi Hospital Jabriya 1828282 25324090 ﻣﺳﺗﺷﻔﻰ ﺍﻟﻌﺭﻑ Al Orf Hospital Al Jahra 2455 5050 2456 7794 ﻣﺳﺗﺷﻔﻰ ﺭﻭﻳﺎﻝ ﺣﻳﺎﺓ Royale Hayat Hospital Jabriya 25360000 25360001 ﻣﺳﺗﺷﻔﻰ ﻋﺎﻟﻳﺔ ﺍﻟﺩﻭﻟﻲ Alia International Hospital Mahboula 22272000 23717020 ﻣﺳﺗﺷﻔﻰ ﺳﺩﺭﺓ Sidra Hospital Al Reggai 24997000 24997070 1881122 ﻣﺳﺗﺷﻔﻰ ﺍﻟﺳﻳﻑ Al Seef Hospital Salmiya 25719810 25764000 25747590 ﻣﺳﺗﺷﻔﻰ ﺍﻟﻣﻭﺍﺳﺎﺓ ﺍﻟﺟﺩﻳﺩ New Mowasat Hospital Salmiya 25726666 25738055 25529012 ﻣﺳﺗﺷﻔﻰ ﻁﻳﺑﺔ Taiba Hospital Sabah Al-Salem 1808088 25528693 ﻣﺳﺗﺷﻔﻰ ﺍﻟﻛﻭﻳﺕ Kuwait Hospital Sabah Al-Salem 22207777 ﻣﺳﺗﺷﻔﻰ ﻭﺍﺭﺓ Wara Hospital Sabah Al-Salem 1888001 ﺍﻟﻣﺳﺗﺷﻔﻰ ﺍﻟﺩﻭﻟﻲ International Hospital Salmiya 1817771 This network is subject to continuous revision by addition/deletion of provider(s) and/or inclusion/exclusion of doctor(s)/department(s) Page 1 of 23 GlobeMed Kuwait Network of HealthCare Providers As of June 2021 Phone ﺍﻻﺳﻡ ProviderProvider Name Name Region Region Phone Number FaxFax Number Number Medical Centers ﻣﺳﺗﻭﺻﻑ ﻣﻳﺩﻳﻛﺎﻝ ﻭﻥ ﺍﻟﺗﺧﺻﺻﻲ Medical one Polyclinic Al Da'iyah 22573883 22574420 1886699 ﻋﻳﺎﺩﺓ ﻧﻭﺭ Noor Clinic Al Ageila 23845957 23845951 22620420 ﻣﺭﻛﺯ ﺍﻟﺷﻌﺏ ﺍﻟﺗﺧﺻﺻﻲ -

Indicators of Urban Health in the Youth Population of Kuwait City and Jahra, Kuwait
Indicators of urban health in the youth population of Kuwait City and Jahra, Kuwait Fayez Alzarban A thesis submitted to the University of Liverpool for the degree of Doctor of Philosophy (Public Health) at the Faculty of Health and Life Sciences March: 2018 Declaration No portion of the work in this thesis has been submitted in support of an application for any degree or qualification of the University of Liverpool or any other University or institute of learning. Signature Acknowledgements I would like to start by thanking my outstanding supervisors at the University of Liverpool: Dr. Daniel Pope and Dr. Debbi Stanistreet, for their constant support, dedication, and encouragement throughout my study period. I am also grateful for all the help I have received from my academic advisors allocated by the Postgraduate team: Prof. Dame Margaret Whitehead, Prof. Sally Sheard, Prof. David Taylor- Robinson, and Prof. Martin O’Flaherty, for all the advice and guidance they have given me. I would also like to thank Prof. Susan Higham for tremendous support at a very difficult period during my studies. In Kuwait, I would like to extend my thanks to Dr. Jafaar Dawood, the head of the Department of Public Health at the Ministry of Health (Kuwait), for his support in every step of this research in Kuwait. Without his exceptional efforts, this project would have not been possible. I would also like to thank the health inspectors at the Department of Health (Kuwait) for their generosity and dedication in conducting the survey with me: Basim Awkal, MS. Allimby, Ala’a Jaad, Saleh Mohammed, Michelle Asaad, Sarah Alazmi, and Hesa Alali. -

Kuwait Institute for Medical Specialization Faculty of Primary Healthcare
Kuwait Institute for Medical Specialization Faculty of Primary Healthcare Family Medicine Residency Program Trainers’ & Residents’ Guide to the curriculum 1 Preface Our vision at The Kuwait Family Medicine Academic Program is to improve the health of the people of Kuwait through leadership in family medicine education, clinical practice, and research. To fulfill this vision, our mission is to develop and maintain exemplary family medicine educational programs for medical students, resident physicians, physician assistants, other faculty and practicing physicians who train healthcare providers for Kuwait. Furthermore, we thrive to provide comprehensive, high quality, cost effective and humanistic healthcare in our family medicine clinical education centers through interdisciplinary cooperation. In our mission we will promote the discovery and dissemination of knowledge that is important to teaching, clinical practice, and organization of healthcare. Finally, we will work in partnership with individuals, community organizations, and governmental institutions to address unmet primary care needs through education, community service, and contributions to help in improving health care delivery systems, while providing a nurturing educational and work environment where creativity is encouraged and diversity is respected. This publication demonstrates the Family Medicine Curriculum in depth for the family medicine trainers, residents, medical students, and other faculty and practicing physicians who train in Family Medicine Centers. Dr. Huda Alduwaisan Chairman of the faculty of primary healthcare 2 Foreword Family Medicine provides accessible, quality and cost-effective healthcare that is patient centered, evidence based, family focused, and problem oriented. Family physicians are expert at managing common complaints, recognizing important diseases, uncovering hidden conditions, and managing most acute and chronic illnesses. -

Kuwait Finance House “KFH” Report on Local Real Estate Market Q4 Year 2017
Kuwait Finance House “KFH” Report On Local Real Estate Market Q4 Year 2017 2 Kuwait Finance House “KFH” Report On Local Real Estate Market Q4 Year 2017 Registered in the Literary Property Sector Bookmark Filing No.: ISBN: 978 - 9921 - 9705 - 0 - 0 Copy or Edition No.: 45 Kuwait Finance House reserves the right to publish the general average area wise depending on several actual indicators and sales as per KFH surveys. It is not permissible to quote, copy or transcript any part of the report wholly or partially without the prior written consent of the publisher or the author (Kuwait Finance House). Violators shall render themselves liable for legal questioning. Kuwait Finance House “KFH” Report On Local Real Estate Market Q4 Year 2017 3 Contents Introduction 6 First: Market Trends 8 Land Prices in Q4 2017 8 Market Trends in Q4 9 Real Estate Trading Trends in Q4 10 Private Residence Trends 11 Investment Real Estate Trends 12 Commercial Real Estate Trends 14 Second: Housing Projects 15 List of Public Authority for Housing Welfare Projects 16 Third: Price Indicators 17 First: Private Residence Land Prices 17 Capital Governorate 17 Hawalli Governorate 18 Farwaniya Governorate 21 Mubarak Al-Kabeer Governorate 22 Ahmadi Governorate 24 Jahra Governorate 25 Second: Investment Land Prices 27 Capital Governorate 27 Hawalli Governorate 28 Farwaniya Governorate 31 Mubarak Al-Kabeer Governorate 32 Ahmadi Governorate 32 Jahra Governorate 34 Annual Rate of Return on Investment Real Estates 34 Third: Commercial Land Prices 35 Capital Governorate 36 Hawalli