10 Recommendations
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
The Ethical Consistency of Animal Equality
1 The ethical consistency of animal equality Stijn Bruers, Sept 2013, DRAFT 2 Contents 0. INTRODUCTION........................................................................................................................................ 5 0.1 SUMMARY: TOWARDS A COHERENT THEORY OF ANIMAL EQUALITY ........................................................................ 9 1. PART ONE: ETHICAL CONSISTENCY ......................................................................................................... 18 1.1 THE BASIC ELEMENTS ................................................................................................................................. 18 a) The input data: moral intuitions .......................................................................................................... 18 b) The method: rule universalism............................................................................................................. 20 1.2 THE GOAL: CONSISTENCY AND COHERENCE ..................................................................................................... 27 1.3 THE PROBLEM: MORAL ILLUSIONS ................................................................................................................ 30 a) Optical illusions .................................................................................................................................... 30 b) Moral illusions .................................................................................................................................... -

Chimpanzee Rights: the Philosophers' Brief
Chimpanzee Rights: The Philosophers’ Brief By Kristin Andrews Gary Comstock G.K.D. Crozier Sue Donaldson Andrew Fenton Tyler M. John L. Syd M Johnson Robert C. Jones Will Kymlicka Letitia Meynell Nathan Nobis David M. Peña-Guzmán Jeff Sebo 1 For Kiko and Tommy 2 Contents Acknowledgments…4 Preface Chapter 1 Introduction: Chimpanzees, Rights, and Conceptions of Personhood….5 Chapter 2 The Species Membership Conception………17 Chapter 3 The Social Contract Conception……….48 Chapter 4 The Community Membership Conception……….69 Chapter 5 The Capacities Conception……….85 Chapter 6 Conclusions……….115 Index 3 Acknowledgements The authors thank the many people who have helped us throughout the development of this book. James Rocha, Bernard Rollin, Adam Shriver, and Rebecca Walker were fellow travelers with us on the amicus brief, but were unable to follow us to the book. Research assistants Andrew Lopez and Caroline Vardigans provided invaluable support and assistance at crucial moments. We have also benefited from discussion with audiences at the Stanford Law School and Dalhousie Philosophy Department Colloquium, where the amicus brief was presented, and from the advice of wise colleagues, including Charlotte Blattner, Matthew Herder, Syl Ko, Tim Krahn, and Gordon McOuat. Lauren Choplin, Kevin Schneider, and Steven Wise patiently helped us navigate the legal landscape as we worked on the brief, related media articles, and the book, and they continue to fight for freedom for Kiko and Tommy, and many other nonhuman animals. 4 1 Introduction: Chimpanzees, Rights, and Conceptions of Personhood In December 2013, the Nonhuman Rights Project (NhRP) filed a petition for a common law writ of habeas corpus in the New York State Supreme Court on behalf of Tommy, a chimpanzee living alone in a cage in a shed in rural New York (Barlow, 2017). -

Trial on Personhood for Chimp Iihias111
~ ~~ -~------------- Trial on personhood for chimp IIHias111 Martin Balluch 1 und Eberhart Theuer' [Verein Gegen Tierfabriken, Vienna, Austria, 2University of Vienna, Austria Summary Zusammenfassung: Gerichtsverfahren zur Erlangung des Perso- In 1982, the chimp "Hiasl" was abducted from the Western nenrechts für den Schimpansen Hiasl African jungle to be used in scientific experiments in Austria. Der Schimpanse "Hiasl" wurde 1982 aus dem Dschungel West- Since his abduction was illegal, he was freed at the airport. Af- afrikas nach Österreich entführt, um hier zu Tierversuchen ver- ter long legal battles with the company responsible for his ab- wendet zu werden. Da es sich um einen illegalen Transport han- duction, he grew up in a humanfamily and now lives at a Vien- delte, wurde er am Flughafen von Zollbeamten befreit. Nach nese anima I shelter. In 2006, this shelter ran into financial langem Rechtsstreit wuchs Hiasl in einer menschlichen Familie difficulties and "Hiasl" was threatened with deportation. und dem Wiener Tierschutzhaus auf Durch finanzielle Schwie- Therefore in 2007, his close [riends started legal procedures to rigkeiten des Tierschutzhauses Ende 2006 war er plötzlich mit have him declared a person and to have a legal guardian ap- Abschiebung ins Ausland bedroht. Deshalb begannen seine pointed for him to represent him in court. Foul' renowned ex- Freunde einen Sachwaltschaftsprozess am Bezirksgericht Möd- perts in anthropology, biology and law supported the case sei- fing für seine Anerkennung als Person. Vier anerkannte Exper- entifically. According to Austrian civil law, all members of the tinnen in Anthropologie, Biologie und Rechtswissenschaften genus "Homo" are persons. Sharing 99.4% of human genes, unterstützten diesen Antrag wissenschaftlich. -

Route 1, Hampton NH • 603.926.8733 • Tile • Tile Stone Floors • • Wood Carpet • Stone Fabrication Eno’S Designing & a 40A | Age P
HAPPY HOLIDAYS! Inside: Seacoast Holidays ALSO INSIDE: 16 VOICES 26,000 COPIES Please Deliver Before FRIDAY, DECEMBER 15, 2006 Vol. 32 | No. 49 | 3 Sections |40 Pages In tune with the School concerts hit a holiday season high note for students Cyan Magenta Yellow Black BY SCOTT E. KINNEY ble treated those in atten- EDITOR, 16 VOICes dance to “Holiday Magic,” a EXETER | The gymna- collection of three Christmas sium of the Lincoln Street tunes and “Jingle March,” Royal Caribbean is currently promoting January and February sailings. School was packed to capac- written by string ensemble Below is a sample of many itineraries being offered at promotional rates. ity on Tuesday night, as the director Nancy McGehee. Prices are cruise only!!! school’s fifth graders treated Not to be outdone the 15-Jan-2007 4 Nt. Bahamas Cruise Sovereign of the Seas their audience to a holiday school’s chorus performed its Inside $244.00 Ocean-view $259.00 WE SPECIALIZE IN SERVICE OF musical extravaganza. version of “Once on a House- 11-Jan-2007 4 Nt. West Caribbean Cruise Enchantment of the Seas The event began with the top,” an international holiday Inside $279.00 Ocean-view $399.00 fifth grade band perform- musical that lends itself to VOLVOS, JAGUARS 04-Feb-2007 6 Nt. Western Caribbean Cruise Jewel of the Seas ing holiday favorites “Sleigh any holiday this time of year, Inside $499.00 Ocean-view $599.00 Ride” and “The Little Drum- regardless of denomination. & HONDAS mer Boy,” along with “We If those in attendance 04-Feb-2007 7 Nt. -

Born Free and Equal? on the Ethical Consistency of Animal Equality
Born free and equal? On the ethical consistency of animal equality Stijn Bruers Proefschrift voorgelegd tot het bekomen van de graad van Doctor in de Moraalwetenschappen Promotor: Prof. dr. Johan Braeckman Promotor Prof. dr. Johan Braeckman Vakgroep Wijsbegeerte en Moraalwetenschap Decaan Prof. dr. Marc Boone Rector Prof. dr. Anne De Paepe Nederlandse vertaling: Vrij en gelijk geboren? Over ethische consistentie en dierenrechten Faculteit Letteren & Wijsbegeerte Stijn Bruers Born free and equal? On the ethical consistency of animal equality Proefschrift voorgelegd tot het behalen van de graad van Doctor in de moraalwetenschappen 2014 Acknowledgements First of all, I would like to thank my supervisor Prof. Dr. Johan Braeckman. He allowed me to explore many new paths in ethics and philosophy and he took time to guide me through the research. His assistance and advice were precious. Second, I owe gratitude to Prof. Dr. Tom Claes and Tim De Smet for comments and Dianne Scatrine and Scott Bell for proofreading. Thanks to Gitte for helping me with the lay-out and cover. Thanks to the University of Ghent for giving opportunities, knowledge and assistance. From all philosophers I know, perhaps Floris van den Berg has ethical ideas closest to mine. I enjoyed our collaboration and meetings with him. Also the many discussions with animal rights activists of Bite Back, with the participants at the International Animal Rights Conferences and Gatherings and with the many meat eaters I encountered during the years allowed me to refine my theories. Furthermore, I am grateful to anonymous reviewers for some useful comments on my research papers. -

Chimeric Brains Consisted of Primatologists and Other Scientists, Ethicists, and Lawyers
Chimeras Definition Ethical Issues Links Suggested Reading End Notes Definition: What are Chimeras? How and why are they produced? Chimeras are animals composed of cells that originate from two (or more) different species. In the research lab, chimeras are created by introducing cells from one species into the developing embryo or fetus of another. (The name chimera comes from Greek mythology and describes a creature with the head of a lion, the body of a goat, and the tail of a serpent). The first chimeras helped scientists understand questions about developmental biology. A sheep-goat chimera, created in 1984, had the head of a goat and the woolly coat of a sheep.i Chicken-quail chimeras have also been successfully developed.ii,iii Sheep-Goat Chimera Courtesy of Dr. Gary B. Anderson -University of California, Davis Now, researchers are developing human-animal chimeras to study disease processes, test new drugs, and develop organs for future transplant patients. The chimeras are produced by introducing human stem cells into developing animal embryos. The following are some of the major projects utilizing human- animal chimeras underway in the U.S and internationally: Sheep-to-humans organ transplant project: At the University of Nevada at Reno, researchers have added human stem cells to sheep fetuses to create chimeras that they hope will someday serve as a reliable source of organs for liver transplant patients. Some sheep now have livers with up to 80% human cells that produce the compounds normally made by human livers. (The cells are not all in distinct liver lobes but spread throughout the tissue. -

Government Proposals for the Regulation of Hybrid and Chimera Embryos
House of Commons Science and Technology Committee Government proposals for the regulation of hybrid and chimera embryos Fifth Report of Session 2006–07 Volume II Oral and written evidence Ordered by The House of Commons to be printed 28 March 2007 HC 272–ll Published on 5 April 2007 by authority of the House of Commons London: The Stationery Office Limited £18.50 The Science and Technology Committee The Science and Technology Committee is appointed by the House of Commons to examine the expenditure, administration and policy of the Office of Science and Innovation and its associated public bodies. Current membership Mr Phil Willis MP (Liberal Democrat, Harrogate and Knaresborough)(Chairman) Adam Afriyie MP (Conservative, Windsor) Mr Robert Flello MP (Labour, Stoke-on-Trent South) Linda Gilroy MP (Labour, Plymouth Sutton) Dr Evan Harris MP (Liberal Democrat, Oxford West & Abingdon) Dr Brian Iddon MP (Labour, Bolton South East) Chris Mole MP (Labour/Co-op, Ipswich) Mr Brooks Newmark MP (Conservative, Braintree) Dr Bob Spink MP (Conservative, Castle Point) Graham Stringer MP (Labour, Manchester, Blackley) Dr Desmond Turner MP (Labour, Brighton Kemptown) Powers The Committee is one of the departmental Select Committees, the powers of which are set out in House of Commons Standing Orders, principally in SO No.152. These are available on the Internet via www.parliament.uk Publications The Reports and evidence of the Committee are published by The Stationery Office by Order of the House. All publications of the Committee (including press notices) are on the Internet at www.parliament.uk/s&tcom A list of Reports from the Committee in this Parliament is included at the back of this volume. -

Of Mice and "Man": Patentability of Genetic Material and the Protection of Intellectual Property Rights
Dalhousie Journal of Legal Studies Volume 18 Article 4 1-1-2009 Of Mice and "Man": Patentability of Genetic Material and the Protection of Intellectual Property Rights Adam Crane Follow this and additional works at: https://digitalcommons.schulichlaw.dal.ca/djls This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 License. Recommended Citation Adam Crane, "Of Mice and "Man": Patentability of Genetic Material and the Protection of Intellectual Property Rights" (2009) 18 Dal J Leg Stud 93. This Article is brought to you for free and open access by the Journals at Schulich Law Scholars. It has been accepted for inclusion in Dalhousie Journal of Legal Studies by an authorized editor of Schulich Law Scholars. For more information, please contact [email protected]. OF MICE AND “MAN”: PATENTABILITY OF GENETIC MATERIAL AND THE PROTECTION OF INTELLECTUAL PROPERTY RIGHTS ADAM CRANE† Te patenting of genetic material raises a host of concerns such as moral and ethical issues, the weakening of the utility requirement and the blockage of downstream research. Tis article examines the patentability of genetic material in the United States, Canada and the European Union. It discusses the advantages and disadvantages of the current patent system in relation to patents on genetic material. Te article concludes with suggestions regarding the protection of intellectual property rights in light of growing concerns. † Adam Crane, B.A. (Acadia), LL.B. Candidate (2010) (Dalhousie). Adam would like to thank Professor Graham Reynolds and Professor Chidi Oguamanam for their inspiration and advice in preparation of this article. He would like to note that any shortcomings of the article are his own. -

EASTER BUFFET KIDS MENU All Specials Subject to Availability
VOLUME 45, NUMBER 12 WEEKOF APRIL 14- 20,2006 P/joto 6y /?£/V/VV SEVERANCE The Relay for Life to support The American Cancer Society came to Bailey's General Store last Thursday and Friday. Volunteer 'celebrity baggers' from the local community raised some $3,400. It was widely regarded as a huge success and it is expected that Bailey's will do it again next year. There's more to the story on page 5. Above, Chamber of Commerce Executive Director Steve Greenstein (a/k/a 'Turtle Man') and Erick Lindblad, executive director of the Sanibel-Captiva Conservation Foundation do their part to help bag and drum up support. INSET: Photo by JENNY BURNHAM. Matthew Marinello is six years old and a cancer survivor since the age of three months, at the first American Cancer Society Relay for Life held on Sanibel Friday, April 7. NDAY NIGHT IS PRIME TIME!! Served with baked Idaho potato & corn on the cob Snow Crab ~ Grouper EVERYDAY! Shrimp Glorious •- Open Mon - Sat @11 am Sunday 9:00am Served with French Fries & corn on the cob 2330 Palm Ridge Rd. Sanibel Island 37 items on the "Consider the Kids" menu. EASTER BUFFET KIDS MENU All specials subject to availability. 37 of Their Favorite Meals Master Card/Visa, Discover Credit Cards Accepted Sunday, April 16 OPEN @ 8 AM Week of April 14 - 20, 2006 ISLANDER Call The Simmons Team - Your Real Estate Leaders ResortQuest Real Estate 239.472.1511 — Office 1019 Periwinkle Way 800.233.8826 -Toll Free Sanibel Island, FL 33957 239.395.9504 - After Hours 239.634.7623-Mobile RESORTQJJEST REAL ESTiTF ^»— ' Glen Simmons, J.D. -
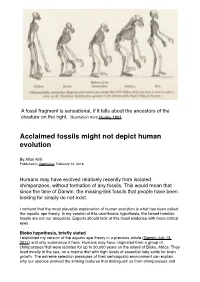
Acclaimed Fossils Might Not Depict Human Evolution
Page 1! A fossil fragment is sensational, if it tells about the ancestors of the creature on the right. Illustration from Huxley 1863 Acclaimed fossils might not depict human evolution By Allan Krill Published in Gemini.no, February 12, 2018 Humans may have evolved relatively recently from isolated chimpanzees, without formation of any fossils. This would mean that since the time of Darwin, the missing-link fossils that people have been looking for simply do not exist. I contend that the most plausible explanation of human evolution is what has been called the aquatic ape theory. In my version of this unorthodox hypothesis, the famed hominin fossils are not our ancestors. Experts should look at this fossil evidence with more critical eyes. Bioko hypothesis, briefly stated I explained my version of the aquatic ape theory in a previous article (Gemini July 13, 2017) and only summarize it here. Humans may have originated from a group of chimpanzees that were isolated for up to 30,000 years on the island of Bioko, Africa. They lived mostly in the sea, on a marine diet with high levels of essential fatty acids for brain growth. The extreme selection pressures of their semiaquatic environment can explain why our species evolved the striking features that distinguish us from chimpanzees and Page 2! other apes, such as our large brain, naked skin, external nose, long legs, long scalp hair, and newborns with weak neck and baby blubber-fat. No fossils would have formed along the coast of Bioko, because it was an erosional geologic environment without deposition of sediments. -

Building Transhuman Immortals-Revised
Histo Possum Tramp Wine Troy Horse Neptune War Roundup History Impossible World Thought Not Bot Wold Roundup Cartoon UBoo-ty Experiments Of The Head History of the West Faerie Tale Fro Gromets Severed From Collections in A Fictional History of the Future Fan Faux NonFic Hypno, Mysto, Crypto Possum Starchitect Kurk Wold 2 I. U-Booty Improvements I went down stairwells into caverns with no markings except codes, wandered, 10, 14 levels below the capitol. The walls went from stone to carved cave rock. Down there they stored artifacts, statues. I was stuck for hours until I found stairways leading up. None of them connected from one floor to the other. I kept walking to an elevator, pushed the up button, found myself in a non-public area. But I had my clearance tag. Asked by security, I told them I was lost. They escorted me to one of the main hallways. I made at least 14 levels below. Passages slope up and down from there where the hearts and livers are stored. I did not ask to investigate the spread of Ubu beyond earth, the beginning of weirdness, as if wierdness had a beginning and we all did not wake up to it individually while others slept to the song of opposite meaning. Rays of light popped off the original incarnation of Gulag and Babel as the same structure pounded itself into the ground. But on the tower, as one climbs higher and higher gravity decreases, and because it is constructed on the equator rotation causes gravitation to 3 disappear because of the distance from the center of the planet, but also from centrifugal force increasing proportionate to that distance. -

THE CHROMOSOME SHUFFLE Activity by Thomas Mueller - RHS (With Reference to Several Articles)
THE CHROMOSOME SHUFFLE Activity by Thomas Mueller - RHS (with reference to several articles) “Creation Scientists” (an oxymoron if ever there was one) suggest that Evolution cannot be correct according to their naive understanding of Genetics. When comparing a karyotype of a chimpanzee (or gorilla) and a karyotype of a human side by side it looks like two ancestral ape chromosomes fused to produce a human total of 46 chromosomes; where gorillas and chimpanzees still have the ancestral 48 chromosomes. A Partial Karyotype: Human Chromosomes 1-4 compared to Chimpanzee chromosomes. (Chromosome on left of each pair is Human; while chromosome(s) on right is/are Chimpanzee) “Creation Scientists” remain unconvinced and argue there could never have been a fusion of two ape-ancestor chromosomes. They argue the karyotypes must have been established this way from the moment of creation. Your task is to understand both arguments by reading the following article: THE CHROMOSOME SHUFFLE (Adapted from Carl Zimmer as posted on his science blog at Discover Magazine) http://blogs.discovermagazine.com/loom/2005/08/29/the-chromosome-shuffle/ Our genes are arrayed along 23 pairs of chromosomes. On rare occasion, a mutation can change their order. If we picture the genes on a chromosome as ABCDEFGHIJKLMNOPQRSTUVWXYZ ...a mutation might flip a segment of the chromosome, so that it now reads ABCDEFGHISRQPONMLKJTUVWXYZ - ...or it might move one segment somewhere else like this: ABCDLMNOPQRSTUEFGHIJKVWXYZ - In some cases, these changes can spread into the genome of an entire species, and be passed down to its descendant species. By comparing the genomes of other mammals to our own, scientists have discovered how the order of our genes has been shuffled over the past 100 million years.