This Article Was Originally Published in a Journal Published by Elsevier
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Trends of Aquatic Alien Species Invasions in Ukraine
Aquatic Invasions (2007) Volume 2, Issue 3: 215-242 doi: http://dx.doi.org/10.3391/ai.2007.2.3.8 Open Access © 2007 The Author(s) Journal compilation © 2007 REABIC Research Article Trends of aquatic alien species invasions in Ukraine Boris Alexandrov1*, Alexandr Boltachev2, Taras Kharchenko3, Artiom Lyashenko3, Mikhail Son1, Piotr Tsarenko4 and Valeriy Zhukinsky3 1Odessa Branch, Institute of Biology of the Southern Seas, National Academy of Sciences of Ukraine (NASU); 37, Pushkinska St, 65125 Odessa, Ukraine 2Institute of Biology of the Southern Seas NASU; 2, Nakhimova avenue, 99011 Sevastopol, Ukraine 3Institute of Hydrobiology NASU; 12, Geroyiv Stalingrada avenue, 04210 Kiyv, Ukraine 4Institute of Botany NASU; 2, Tereschenkivska St, 01601 Kiyv, Ukraine E-mail: [email protected] (BA), [email protected] (AB), [email protected] (TK, AL), [email protected] (PT) *Corresponding author Received: 13 November 2006 / Accepted: 2 August 2007 Abstract This review is a first attempt to summarize data on the records and distribution of 240 alien species in fresh water, brackish water and marine water areas of Ukraine, from unicellular algae up to fish. A checklist of alien species with their taxonomy, synonymy and with a complete bibliography of their first records is presented. Analysis of the main trends of alien species introduction, present ecological status, origin and pathways is considered. Key words: alien species, ballast water, Black Sea, distribution, invasion, Sea of Azov introduction of plants and animals to new areas Introduction increased over the ages. From the beginning of the 19th century, due to The range of organisms of different taxonomic rising technical progress, the influence of man groups varies with time, which can be attributed on nature has increased in geometrical to general processes of phylogenesis, to changes progression, gradually becoming comparable in in the contours of land and sea, forest and dimensions to climate impact. -

Aquatic Molluscs of the Mrežnica River
NAT. CROAT. VOL. 28 No 1 99-106 ZAGREB June 30, 2019 original scientific paper / izvorni znanstveni rad DOI 10.20302/NC.2019.28.9 AQUATIC MOLLUSCS OF THE MREŽNICA RIVER Luboš Beran Nature Conservation Agency of the Czech Republic, Regional Office Kokořínsko – Máchův kraj Protected Landscape Area Administration, Česká 149, CZ–276 01 Mělník, Czech Republic (e-mail: [email protected]) Beran, L.: Aquatic molluscs of the Mrežnica River. Nat. Croat. Vol. 28, No. 1., 99-106, Zagreb, 2019. Results of a malacological survey of the Mrežnica River are presented. The molluscan assemblages of this river between the boundary of the Eugen Kvarternik military area and the inflow to the Korana River near Karlovac were studied from 2013 to 2018. Altogether 29 aquatic molluscs (19 gastropods, 10 bivalves) were found at 9 sites. Theodoxus danubialis, Esperiana esperi, Microcolpia daudebartii and Holandriana holandrii were dominant at most of the sites. The molluscan assemblages were very similar to assemblages documented during previous research into the Korana River. The populations of the endangered bivalves Unio crassus and Pseudanodonta complanata were recorded. An extensive population of another endangered gastropod Anisus vorticulus was found at one site. Physa acuta is the only non-native species confirmed in the Mrežnica River. Key words: Mollusca, Unio crassus, Anisus vorticulus, Mrežnica, faunistic, Croatia Beran, L.: Vodeni mekušci rijeke Mrežnice. Nat. Croat. Vol. 28, No. 1., 99-106, Zagreb, 2019. U radu su predstavljeni rezultati malakološkog istraživanja rijeke Mrežnice. Sastav mekušaca ove rijeke proučavan je na području od Vojnog poligona Eugen Kvarternik do njenog utoka u Koranu blizu Karlovca, u razdoblju 2013. -

A Conservation Palaeobiological Approach to Assess Faunal Response of Threatened Biota Under Natural and Anthropogenic Environmental Change
Biogeosciences, 16, 2423–2442, 2019 https://doi.org/10.5194/bg-16-2423-2019 © Author(s) 2019. This work is distributed under the Creative Commons Attribution 4.0 License. A conservation palaeobiological approach to assess faunal response of threatened biota under natural and anthropogenic environmental change Sabrina van de Velde1,*, Elisabeth L. Jorissen2,*, Thomas A. Neubauer1,3, Silviu Radan4, Ana Bianca Pavel4, Marius Stoica5, Christiaan G. C. Van Baak6, Alberto Martínez Gándara7, Luis Popa7, Henko de Stigter8,2, Hemmo A. Abels9, Wout Krijgsman2, and Frank P. Wesselingh1 1Naturalis Biodiversity Center, P.O. Box 9517, 2300 RA Leiden, the Netherlands 2Palaeomagnetic Laboratory “Fort Hoofddijk”, Faculty of Geosciences, Utrecht University, Budapestlaan 17, 3584 CD Utrecht, the Netherlands 3Department of Animal Ecology & Systematics, Justus Liebig University, Heinrich-Buff-Ring 26–32 IFZ, 35392 Giessen, Germany 4National Institute of Marine Geology and Geoecology (GeoEcoMar), 23–25 Dimitrie Onciul St., 024053 Bucharest, Romania 5Department of Geology, Faculty of Geology and Geophysics, University of Bucharest, Balcescu˘ Bd. 1, 010041 Bucharest, Romania 6CASP, West Building, Madingley Rise, Madingley Road, CB3 0UD, Cambridge, UK 7Grigore Antipa National Museum of Natural History, Sos. Kiseleff Nr. 1, 011341 Bucharest, Romania 8NIOZ Royal Netherlands Institute for Sea Research, Department of Ocean Systems, 1790 AB Den Burg, the Netherlands 9Department of Geosciences and Engineering, Delft University of Technology, Stevinweg 1, 2628 CN Delft, the Netherlands *These authors contributed equally to this work. Correspondence: Sabrina van de Velde ([email protected]) and Elisabeth L. Jorissen ([email protected]) Received: 10 January 2019 – Discussion started: 31 January 2019 Revised: 26 April 2019 – Accepted: 16 May 2019 – Published: 17 June 2019 Abstract. -

The Relationship Between Aquatic Macrophytes and Some Gastropoda (Snails) in the Lower Reaches of Hammar Marsh Inaam A
Mesopotamia Environmental Journal ISSN 2410-2598 Mesop. environ. j. 2016, Vol.2, No.4: 23-32. The relationship between aquatic macrophytes and some gastropoda (snails) in the lower reaches of Hammar marsh Inaam A. A. Qazar Department of Ecology, College of Science, Basrah University Corresponding author:[email protected] To cite this article: Qazar, I.A.A. The relationship between aquatic macrophytes and some gastropoda (snails) in the lower reaches of Hammar marsh. Mesop. environ. j., 2016, Vol.2. ,No.4 ,pp.23-32. Received Date : 14 / 5 / 2016 Accepted Date : 10 / 6 / 2016 Publishing Date : 15/8/2016 This work is licensed under a Creative Commons Attribution- NonCommercial-NoDerivatives 4.0 International License. Abstract This study was carried out during 2011-2012 to evaluate the preference of some freshwater snails to specific macrophytes than others. Snails were collected from different aquatic plants; Ceratophyllum demersum, Potamogeton crispus, Salvinia natans,and Hydrilla verticillata at four stations. The presence of these plants was affected by the water temperature, they almost disappear in winter leading to low snail numbers at that season. Eight snail species were found at the study area; Bellamya bengalensis (Lamarck, 1822), Bithynia hareerensis Glöer, and Nasser, 2008 ,Gyrauluse hrenbergi (Beck, 1837), Melanoides tuberculata (Müller, 1774), Melanopsis nodosa Férussac, 1823, Physlla acuta Draparnaud, 1805, Radix auricularia (Linnaeus,1758), Theodoxus jordani (Sowerby, 1832). Statistical analysis shows a significant differences (P<0.05) between macrophyte's snails number, C. demersum and H. verticilata was the most preferred macrophyte in this study, while S. natanus recorded no occurrence of snails. Keywords:Snail, Macrophytes, Periphyton, Occurance, Morphology. -
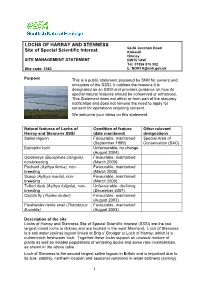
Lochs of Harray and Stenness Site of Special Scientific Interest (SSSI) Are the Two Largest Inland Lochs in Orkney and Are Located in the West Mainland
LOCHS OF HARRAY AND STENNESS 54-56 Junction Road Site of Special Scientific Interest Kirkwall Orkney SITE MANAGEMENT STATEMENT KW15 1AW Tel: 01856 875 302 Site code: 1083 E: [email protected] Purpose This is a public statement prepared by SNH for owners and occupiers of the SSSI. It outlines the reasons it is designated as an SSSI and provides guidance on how its special natural features should be conserved or enhanced. This Statement does not affect or form part of the statutory notification and does not remove the need to apply for consent for operations requiring consent. We welcome your views on this statement. Natural features of Lochs of Condition of feature Other relevant Harray and Stenness SSSI (date monitored) designations Saline lagoon Favourable, maintained Special Area of (September 1999) Conservation (SAC) Eutrophic loch Unfavourable, no change (August 2004) Goldeneye (Bucephala clangula), Favourable, maintained non-breeding (March 2000) Pochard (Aythya ferina), non- Favourable, maintained breeding (March 2000) Scaup (Aythya marila), non- Favourable, maintained breeding (March 2000) Tufted duck (Aythya fuligula), non- Unfavourable, declining breeding (December 2007) Caddis fly (Ylodes reuteri) Favourable, maintained (August 2003) Freshwater nerite snail (Theodoxus Favourable, maintained fluviatilis) (August 2003) Description of the site Lochs of Harray and Stenness Site of Special Scientific Interest (SSSI) are the two largest inland lochs in Orkney and are located in the west Mainland. Loch of Stenness is a salt water (saline) lagoon linked at Brig o’ Brodgar to Loch of Harray, which is a nutrient-rich freshwater loch. Together these lochs support an unusual mixture of plants as well as notable populations of wintering ducks and some rare invertebrates, as shown in the above table. -

A Stable Temperature May Favour Continuous Reproduction by Theodoxus fluviatilis and Explain Its High Densities in Some Karstic Springs
Limnetica, 29 (2): x-xx (2011) Limnetica, 31 (1): 129-140 (2012). DOI: 10.23818/limn.31.12 c Asociaci´on Ib´erica de Limnologı´a, Madrid. Spain. ISSN: 0213-8409 A stable temperature may favour continuous reproduction by Theodoxus fluviatilis and explain its high densities in some karstic springs Manuel A. S. Grac¸a∗,Sonia´ R. Q. Serra and Veronica´ Ferreira IMAR-CMA and Department of Life Sciences, University of Coimbra, PO Box 3046, 3001-401 Coimbra, Portugal. ∗ Corresponding author: [email protected] 2 Received: 30/5/2011 Accepted: 27/12/2011 ABSTRACT A stable temperature may favour continuous reproduction by Theodoxus fluviatilis and explain its high densities in some karstic springs Theodoxus fluviatilis is a common gastropod in many karstic springs in central Portugal. We investigated the possible reasons for the near-total restriction of this species to these springs. We first determined the spatial distribution of the species within a spring (Anc¸os) and related the densities at sampling-patch scales to selected physical and chemical variables. We then determined the densities at several locations downstream from the spring and related these densities to selected physical and chemical variables. Finally, we assessed the population dynamics of the gastropod in the spring. In the spring, T. fluviatilis was more abundant in shallow areas with a rapid current and cobble-boulder substrates. In June-July 2006, the mean densities of T. fluviatilis in the spring varied from ∼ 10 to ∼ 9000 individuals m–2 but decreased to zero 3800 m downstream. The physical and chemical changes along the stretch studied were minor; no significant correlations (Spearman rank correlation; p > 0.05) were observed between the gastropod abundances and the measured environmental variables or the PCA axes. -

The Morphological Plasticity of Theodoxus Fluviatilis (Linnaeus, 1758) (Mollusca: Gastropoda: Neritidae)
Correspondence ISSN 2336-9744 (online) | ISSN 2337-0173 (print) The journal is available on line at www.ecol-mne.com The morphological plasticity of Theodoxus fluviatilis (Linnaeus, 1758) (Mollusca: Gastropoda: Neritidae) PETER GLÖER 1 and VLADIMIR PEŠI Ć2 1 Biodiversity Research Laboratory, Schulstraße 3, D-25491 Hetlingen, Germany. E-mail: [email protected] 2 Department of Biology, Faculty of Sciences, University of Montenegro, Cetinjski put b.b., 81000 Podgorica, Montenegro. E-mail: [email protected] Received 19 January 2015 │ Accepted 3 February 2015 │ Published online 4 February 2015. Theodoxus fluviatilis is a widely distributed species, ranging from the Ireland (Lucey et al . 1992) to Iran (Glöer & Peši ć 2012). The records from Africa were considered as doubtful (Brown 2002), but recently the senior author found this species in Algeria (Glöer unpublished record). The species prefers the lowlands (in Switzerland up to 275 m a.s.l., Turner et al . 1998), and calcium-rich waters, living on hard benthic substrates, typically rocks. On the territory of the Central and Eastern Balkans three species are present (Markovi ć et al . 2014): Theodoxus fluviatilis (Linnaeus, 1758), T. danubialis (C. Pfeiffer, 1828) and T. transversalis (C.Pfeiffer, 1828). For Montenegro, Wohlberedt (1909), Karaman & Karaman (2007), Glöer & Peši ć (2008), and Peši ć & Glöer (2013) listed only Theodoxus fluviatilis . Figures 1-4. The operculum. 1: Theodoxus fluviatilis (Linnaeus, 1758); 2: T. danubialis (C. Pfeiffer, 1828); 3-4: Rib shield of T. fluviatilis . Abbreviations: ca = callus, eo = embryonic operculum, la = left adductor, p = peg, r = rib, ra = right adductor, rp = rib pit, rs = rib shield. The shell of adults of Theodoxus fluviatilis is 4.5–6.5 mm high and 6–9 mm broad, exceptionally up to 13 mm, and consists of 3–3.5 whorls, including protoconch, with a usually low spire. -

Fossil Flora and Fauna of Bosnia and Herzegovina D Ela
FOSSIL FLORA AND FAUNA OF BOSNIA AND HERZEGOVINA D ELA Odjeljenje tehničkih nauka Knjiga 10/1 FOSILNA FLORA I FAUNA BOSNE I HERCEGOVINE Ivan Soklić DOI: 10.5644/D2019.89 MONOGRAPHS VOLUME LXXXIX Department of Technical Sciences Volume 10/1 FOSSIL FLORA AND FAUNA OF BOSNIA AND HERZEGOVINA Ivan Soklić Ivan Soklić – Fossil Flora and Fauna of Bosnia and Herzegovina Original title: Fosilna flora i fauna Bosne i Hercegovine, Sarajevo, Akademija nauka i umjetnosti Bosne i Hercegovine, 2001. Publisher Academy of Sciences and Arts of Bosnia and Herzegovina For the Publisher Academician Miloš Trifković Reviewers Dragoljub B. Đorđević Ivan Markešić Editor Enver Mandžić Translation Amra Gadžo Proofreading Amra Gadžo Correction Sabina Vejzagić DTP Zoran Buletić Print Dobra knjiga Sarajevo Circulation 200 Sarajevo 2019 CIP - Katalogizacija u publikaciji Nacionalna i univerzitetska biblioteka Bosne i Hercegovine, Sarajevo 57.07(497.6) SOKLIĆ, Ivan Fossil flora and fauna of Bosnia and Herzegovina / Ivan Soklić ; [translation Amra Gadžo]. - Sarajevo : Academy of Sciences and Arts of Bosnia and Herzegovina = Akademija nauka i umjetnosti Bosne i Hercegovine, 2019. - 861 str. : ilustr. ; 25 cm. - (Monographs / Academy of Sciences and Arts of Bosnia and Herzegovina ; vol. 89. Department of Technical Sciences ; vol. 10/1) Prijevod djela: Fosilna flora i fauna Bosne i Hercegovine. - Na spor. nasl. str.: Fosilna flora i fauna Bosne i Hercegovine. - Bibliografija: str. 711-740. - Registri. ISBN 9958-501-11-2 COBISS/BIH-ID 8839174 CONTENTS FOREWORD ........................................................................................................... -

From the Iberian Peninsula
Ecologica Montenegrina 18: 133-137 (2018) This journal is available online at: www.biotaxa.org/em On the identity of Neritina baetica Lamarck, 1822 and Nerita meridionalis Philippi, 1836 (Gastropoda: Neritidae) from the Iberian Peninsula PETER GLÖER Biodiversity Research Laboratory, Schulstraße 3, D-25491 Hetlingen, Germany, e-mail: [email protected] Received 2 August 2018 │ Accepted by V. Pešić: 29 August 2018 │ Published online 1 September 2018. Abstract When Mermod (1953) depicted the types of Theodoxus baeticus of Lamarck’s collection his photo did not display a pseudo-apophysis (peg). This led recent authors believe, that Th. baeticus is a synonym of Th. fluviatilis and Theodoxus meridionalis is the valid species (Alba et al. 2016: 55). In this paper I studied the syntypes of Th. baeticus as well as the syntypes of Th. meridionalis and demonstrated that in Th. baeticus a pseudo-apophysis exists and the opercula of Th. baeticus and Th. meridionalis are similar. Thus Th. baeticus is a good species and Th. meridionalis is a younger synonym of it. Therefore I can conclude that three Theodoxus spp., i.e., Th. fluviatilis, Th. baeticus and Th. valentinus occur in the Iberian Peninsula. Key words: Theodoxus baeticus, Theodoxus meridionalis, Iberian Peninsula, syntypes. Introduction Many species have formerly been described on the basis of shell morphology and especially on the patterns or the color of the shells. Many of these nominal taxa belong at least to Theodoxus fluviatilis. For instance in Central Europe Th. fluviatilis has patterns of drop shape, while it has in Spain zigzag lines, and in the Balkans we find a combination of both patterns (Glöer & Pešić 2015). -

Research Article ISSN 2336-9744 (Online) | ISSN 2337-0173 (Print) the Journal Is Available on Line At
Research Article ISSN 2336-9744 (online) | ISSN 2337-0173 (print) The journal is available on line at www.ecol-mne.com http://zoobank.org/urn:lsid:zoobank.org:pub:C19F66F1-A0C5-44F3-AAF3-D644F876820B Description of a new subterranean nerite: Theodoxus gloeri n. sp. with some data on the freshwater gastropod fauna of Balıkdamı Wetland (Sakarya River, Turkey) DENIZ ANIL ODABAŞI1* & NAIME ARSLAN2 1 Çanakkale Onsekiz Mart University, Faculty Marine Science Technology, Marine and Inland Sciences Division, Çanakkale, Turkey. E-mail: [email protected] 2 Eskişehir Osman Gazi University, Science and Art Faculty, Biology Department, Eskişehir, Turkey. E-mail: [email protected] *Corresponding author Received 1 June 2015 │ Accepted 17 June 2015 │ Published online 20 June 2015. Abstract In the present study, conducted between 2001 and 2003, four taxa of aquatic gastropoda were identified from the Balıkdamı Wetland. All the species determined are new records for the study area, while one species Theodoxus gloeri sp. nov. is new to science. Neritidae is a representative family of an ancient group Archaeogastropoda, among Gastropoda. Theodoxus is a freshwater genus in the Neritidae, known for a dextral, rapidly grown shell ended with a large last whorl and a lunate calcareous operculum. Distribution of this genus includes Europe, also extending from North Africa to South Iran. In Turkey, 14 modern and fossil species and subspecies were mentioned so far. In this study, we aimed to uncover the gastropoda fauna of an important Wetland and describe a subterranean Theodoxus species, new to science. Key words: Gastropoda, Theodoxus gloeri sp. nov., Sakarya River, Balıkdamı Wetland Turkey. -

MOLECULAR PHYLOGENY of the NERITIDAE (GASTROPODA: NERITIMORPHA) BASED on the MITOCHONDRIAL GENES CYTOCHROME OXIDASE I (COI) and 16S Rrna
ACTA BIOLÓGICA COLOMBIANA Artículo de investigación MOLECULAR PHYLOGENY OF THE NERITIDAE (GASTROPODA: NERITIMORPHA) BASED ON THE MITOCHONDRIAL GENES CYTOCHROME OXIDASE I (COI) AND 16S rRNA Filogenia molecular de la familia Neritidae (Gastropoda: Neritimorpha) con base en los genes mitocondriales citocromo oxidasa I (COI) y 16S rRNA JULIAN QUINTERO-GALVIS 1, Biólogo; LYDA RAQUEL CASTRO 1,2 , Ph. D. 1 Grupo de Investigación en Evolución, Sistemática y Ecología Molecular. INTROPIC. Universidad del Magdalena. Carrera 32# 22 - 08. Santa Marta, Colombia. [email protected]. 2 Programa Biología. Universidad del Magdalena. Laboratorio 2. Carrera 32 # 22 - 08. Sector San Pedro Alejandrino. Santa Marta, Colombia. Tel.: (57 5) 430 12 92, ext. 273. [email protected]. Corresponding author: [email protected]. Presentado el 15 de abril de 2013, aceptado el 18 de junio de 2013, correcciones el 26 de junio de 2013. ABSTRACT The family Neritidae has representatives in tropical and subtropical regions that occur in a variety of environments, and its known fossil record dates back to the late Cretaceous. However there have been few studies of molecular phylogeny in this family. We performed a phylogenetic reconstruction of the family Neritidae using the COI (722 bp) and the 16S rRNA (559 bp) regions of the mitochondrial genome. Neighbor-joining, maximum parsimony and Bayesian inference were performed. The best phylogenetic reconstruction was obtained using the COI region, and we consider it an appropriate marker for phylogenetic studies within the group. Consensus analysis (COI +16S rRNA) generally obtained the same tree topologies and confirmed that the genus Nerita is monophyletic. The consensus analysis using parsimony recovered a monophyletic group consisting of the genera Neritina , Septaria , Theodoxus , Puperita , and Clithon , while in the Bayesian analyses Theodoxus is separated from the other genera. -

Theodoxus Fluviatilis L., 1758) in the Largest Central European Shallow Lake, Lake Balaton, Hungary
BioInvasions Records (2019) Volume 8, Issue 2: 273–280 CORRECTED PROOF Rapid Communication “Invasion in progress”: first occurrence and spread of river nerite (Theodoxus fluviatilis L., 1758) in the largest Central European shallow lake, Lake Balaton, Hungary Péter Takács1,*, András Ács2, Bálint Bánó3, István Czeglédi1, Judit Csaba4, Tibor Erős1, Melinda Fésűs-Móré5, Bálint Preiszner1, Ádám Staszny2, Zoltán Vitál1, András Weiperth6 and Árpád Ferincz2 1MTA Centre for Ecological Research, Balaton Limnological Institute, Klebelsberg Kuno str. 3., H-8237, Tihany, Hungary 2Szent István University, Department of Aquaculture, Páter Károly str. 1., H-2100, Gödöllő, Hungary 3Pannon University, Georgikon Faculty of Agriculture, Deák Ferenc str. 16., H-8360, Keszthely, Hungary 4Szent István University, Doctoral School of Biological Sciences, Páter Károly str. 1., H-2100, Gödöllő, Hungary 5Government Office of Fejér County, Major Department of Public Health, Laboratory Department, Hosszúsétatér 1., H-8000 Székesfehérvár, Hungary 6MTA Centre for Ecological Research, Danube Research Institute, Karolina str. 29. H-1113, Budapest, Hungary Author e-mails: [email protected] (PT), [email protected] (AÁ), [email protected] (BB), [email protected] (IC), [email protected] (JCs), [email protected] (TE), [email protected] (MFM), [email protected] (BP), [email protected] (ÁS), [email protected] (ZV), [email protected] (AW), [email protected] (ÁF) *Corresponding author Citation: Takács P, Ács A, Bánó B, Czeglédi I, Csaba J, Erős T, Fésűs-Móré Abstract M, Preiszner B, Staszny Á, Vitál Z, Weiperth A, Ferincz Á (2019) “Invasion in This study presents the first occurrence and recent distribution pattern of the river progress”: first occurrence and spread of nerite (Theodoxus fluviatilis L., 1758) in Lake Balaton.