Specific Immune- Related Manifestations of COVID-19
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Syphilis Staging and Treatment Syphilis Is a Sexually Transmitted Disease (STD) Caused by the Treponema Pallidum Bacterium
Increasing Early Syphilis Cases in Illinois – Syphilis Staging and Treatment Syphilis is a sexually transmitted disease (STD) caused by the Treponema pallidum bacterium. Syphilis can be separated into four different stages: primary, secondary, early latent, and late latent. Ocular and neurologic involvement may occur during any stage of syphilis. During the incubation period (time from exposure to clinical onset) there are no signs or symptoms of syphilis, and the individual is not infectious. Incubation can last from 10 to 90 days with an average incubation period of 21 days. During this period, the serologic testing for syphilis will be non-reactive but known contacts to early syphilis (that have been exposed within the past 90 days) should be preventatively treated. Syphilis Stages Primary 710 (CDC DX Code) Patient is most infectious Chancre (sore) must be present. It is usually marked by the appearance of a single sore, but multiple sores are common. Chancre appears at the spot where syphilis entered the body and is usually firm, round, small, and painless. The chancre lasts three to six weeks and will heal without treatment. Without medical attention the infection progresses to the secondary stage. Secondary 720 Patient is infectious This stage typically begins with a skin rash and mucous membrane lesions. The rash may manifest as rough, red, or reddish brown spots on the palms of the hands, soles of the feet, and/or torso and extremities. The rash does usually does not cause itching. Rashes associated with secondary syphilis can appear as the chancre is healing or several weeks after the chancre has healed. -

Dermatology DDX Deck, 2Nd Edition 65
63. Herpes simplex (cold sores, fever blisters) PREMALIGNANT AND MALIGNANT NON- 64. Varicella (chicken pox) MELANOMA SKIN TUMORS Dermatology DDX Deck, 2nd Edition 65. Herpes zoster (shingles) 126. Basal cell carcinoma 66. Hand, foot, and mouth disease 127. Actinic keratosis TOPICAL THERAPY 128. Squamous cell carcinoma 1. Basic principles of treatment FUNGAL INFECTIONS 129. Bowen disease 2. Topical corticosteroids 67. Candidiasis (moniliasis) 130. Leukoplakia 68. Candidal balanitis 131. Cutaneous T-cell lymphoma ECZEMA 69. Candidiasis (diaper dermatitis) 132. Paget disease of the breast 3. Acute eczematous inflammation 70. Candidiasis of large skin folds (candidal 133. Extramammary Paget disease 4. Rhus dermatitis (poison ivy, poison oak, intertrigo) 134. Cutaneous metastasis poison sumac) 71. Tinea versicolor 5. Subacute eczematous inflammation 72. Tinea of the nails NEVI AND MALIGNANT MELANOMA 6. Chronic eczematous inflammation 73. Angular cheilitis 135. Nevi, melanocytic nevi, moles 7. Lichen simplex chronicus 74. Cutaneous fungal infections (tinea) 136. Atypical mole syndrome (dysplastic nevus 8. Hand eczema 75. Tinea of the foot syndrome) 9. Asteatotic eczema 76. Tinea of the groin 137. Malignant melanoma, lentigo maligna 10. Chapped, fissured feet 77. Tinea of the body 138. Melanoma mimics 11. Allergic contact dermatitis 78. Tinea of the hand 139. Congenital melanocytic nevi 12. Irritant contact dermatitis 79. Tinea incognito 13. Fingertip eczema 80. Tinea of the scalp VASCULAR TUMORS AND MALFORMATIONS 14. Keratolysis exfoliativa 81. Tinea of the beard 140. Hemangiomas of infancy 15. Nummular eczema 141. Vascular malformations 16. Pompholyx EXANTHEMS AND DRUG REACTIONS 142. Cherry angioma 17. Prurigo nodularis 82. Non-specific viral rash 143. Angiokeratoma 18. Stasis dermatitis 83. -

Skin Manifestation of SARS-Cov-2: the Italian Experience
Journal of Clinical Medicine Article Skin Manifestation of SARS-CoV-2: The Italian Experience Gerardo Cazzato 1 , Caterina Foti 2, Anna Colagrande 1, Antonietta Cimmino 1, Sara Scarcella 1, Gerolamo Cicco 1, Sara Sablone 3, Francesca Arezzo 4, Paolo Romita 2, Teresa Lettini 1 , Leonardo Resta 1 and Giuseppe Ingravallo 1,* 1 Section of Pathology, University of Bari ‘Aldo Moro’, 70121 Bari, Italy; [email protected] (G.C.); [email protected] (A.C.); [email protected] (A.C.); [email protected] (S.S.); [email protected] (G.C.); [email protected] (T.L.); [email protected] (L.R.) 2 Section of Dermatology and Venereology, University of Bari ‘Aldo Moro’, 70121 Bari, Italy; [email protected] (C.F.); [email protected] (P.R.) 3 Section of Forensic Medicine, University of Bari ‘Aldo Moro’, 70121 Bari, Italy; [email protected] 4 Section of Gynecologic and Obstetrics Clinic, University of Bari ‘Aldo Moro’, 70121 Bari, Italy; [email protected] * Correspondence: [email protected] Abstract: At the end of December 2019, a new coronavirus denominated Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) was identified in Wuhan, Hubei province, China. Less than three months later, the World Health Organization (WHO) declared coronavirus disease-19 (COVID-19) to be a global pandemic. Growing numbers of clinical, histopathological, and molecular findings were subsequently reported, among which a particular interest in skin manifestations during the course of the disease was evinced. Today, about one year after the development of the first major infectious foci in Italy, various large case series of patients with COVID-19-related skin Citation: Cazzato, G.; Foti, C.; manifestations have focused on skin specimens. -
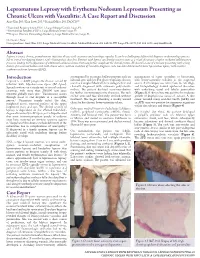
Lepromatous Leprosy with Erythema Nodosum Leprosum Presenting As
Lepromatous Leprosy with Erythema Nodosum Leprosum Presenting as Chronic Ulcers with Vasculitis: A Case Report and Discussion Anny Xiao, DO,* Erin Lowe, DO,** Richard Miller, DO, FAOCD*** *Traditional Rotating Intern, PGY-1, Largo Medical Center, Largo, FL **Dermatology Resident, PGY-2, Largo Medical Center, Largo, FL ***Program Director, Dermatology Residency, Largo Medical Center, Largo, FL Disclosures: None Correspondence: Anny Xiao, DO; Largo Medical Center, Graduate Medical Education, 201 14th St. SW, Largo, FL 33770; 510-684-4190; [email protected] Abstract Leprosy is a rare, chronic, granulomatous infectious disease with cutaneous and neurologic sequelae. It can be a challenging differential diagnosis in dermatology practice due to several overlapping features with rheumatologic disorders. Patients with leprosy can develop reactive states as a result of immune complex-mediated inflammatory processes, leading to the appearance of additional cutaneous lesions that may further complicate the clinical picture. We describe a case of a woman presenting with a long history of a recurrent bullous rash with chronic ulcers, with an evolution of vasculitic diagnoses, who was later determined to have lepromatous leprosy with reactive erythema nodosum leprosum (ENL). Introduction accompanied by an intense bullous purpuric rash on management of sepsis secondary to bacteremia, Leprosy is a slowly progressive disease caused by bilateral arms and face. For these complaints she was with lower-extremity cellulitis as the suspected infection with Mycobacterium leprae (M. leprae). seen in a Complex Medical Dermatology Clinic and source. A skin biopsy was taken from the left thigh, Spread continues at a steady rate in several endemic clinically diagnosed with cutaneous polyarteritis and histopathology showed epidermal ulceration countries, with more than 200,000 new cases nodosa. -

Understanding Vasculitis Factsheet
Vasculitis - The Facts This factsheet is intended as a simple There is no cure for PSV, but it can usually be introduction to vasculitis for those who have just controlled by use of steroids, chemotherapy and been diagnosed with vasculitis, members of their immune suppressing drugs. Long term drug therapy is family, friends, work colleagues and for others often required. If all goes well some patients go into who may want to know about the disease. “full remission” - ie they no longer need drugs. But relapse is common. What is Vasculitis Vasculitis is a rare inflammatory disease which affects People suffering from vasculitis often experience about 2-3000 new people each year in the UK. muscle weakness and chronic fatigue. Some Vasculitis means inflammation of the blood vessels. experience chronic pain due to nerve damage or Any vessels in any part of the body can be affected. severe migraines and headaches due to damaged blood vessels in the head. There are several different types of vasculitis. In the first type, the acute form, it can be caused by Others require dialysis or kidney transplants. Many infections, reaction to drugs or exposure to chemicals. have breathing problems and others are left with Often the problem is localised, such as a rash. In these permanent physical disabilities. cases the disease usually needs no treatment. Other types of vasculitis can be secondary to (or as a The most “common” types of these rare consequence of) other illnesses such as rheumatoid vasculitis diseases are: arthritis or some types of cancer. ° Granulomatosis with Polyangiitis (GPA) (previously known as Wegener’s Granulomatosis) The third group is known as Primary Systemic ° Eosinophilic Granulomatosis with Polyangiitis (EGPA) Vasculitis (PSV). -

Erythema Nodosum (En)
Buffalo Medical Group, P.C. Robert E. Kalb, M.D. Phone: (716) 630-1102 Fax: (716) 633-6507 Department of Dermatology 325 Essjay Road Williamsville, New York 14221 ERYTHEMA NODOSUM (EN) Erythema Nodosum (EN) is a relatively uncommon reaction in the skin consisting of red shiny tender nodular lesions most commonly found on the legs. The erythema refers to the red color that is seen at the surface of the skin. The nodosum refers to the fact that these lesions feel like bumps or nodules underneath the skin surface. Erythema nodosum is a reaction pattern occurring in the subcutaneous tissue and fat. It may be preceded by a low grade fever, fatigue, and joint pains. In about half of the cases, there is an internal condition occurring in the body which contributes to its formation. These include the use of certain medications, certain infections, and a number of other less likely causes. In order to identify cause for the erythema nodosum, it may be necessary to perform blood and laboratory tests. At times, however, the cause cannot be determined and erythema nodosum is considered to be idiopathic in nature. Erythema nodosum often runs an acute course lasting for a brief period from weeks to months. In a small percentage of cases, it can be a more chronic condition. The red nodules are usually confined to the lower legs, but can develop anywhere there is fat under the skin including the thighs, arms, and trunk. Initially, the lesions are more red and inflamed in appearance, but with time become more bruise like. -

MYELOPATHY ASSOCIATED with SYSTEMIC LUPUS ERYTHEMATOSUS (Erythema Nodosum)
Paraplegia 16 (1978-79) 282-294 Original Articles MYELOPATHY ASSOCIATED WITH SYSTEMIC LUPUS ERYTHEMATOSUS (Erythema Nodosum) L. S. KEWALRAMANI, M.D., M.S.Orth., S. SALEEM, M.D. and D. BERTRAND, M.T. (ASCP) Texas Institute for Rehabilitation and Research and Department of Pathology, Baylor College of Medicine, Houston, Texas 77030, U.S.A. Abstract. Two patients with sudden onset of myelopathy associated with Systemic Lupus Erythematosus (Erythema Nodosum) are described. Pertinent literature is extensively reviewed and these two new patients are added to previously reported 26 patients. Key words: Myelopathy; Meningoencephalomyelopathy; Systemic lupus erythematosus. NEUROLOGICAL manifestations of systemic lupus erythematosus (SLE) have only recently been emphasised although they were mentioned by Kaposi in 1875, who observed stupor and coma as terminal manifestations of the disease. But focal neurological abnormalities were first reported by Osler (1903) and since then there have been several reports in the literature. Most commonly reported entities have been acute organic brain syndrome, seizures and cerebrovascular disorders. Chorea, Guillain Barre syndrome, subarachnoid haemorrhage, peripheral neuro pathy and cranial nerve palsies associated with SLE have also been reported on a few occasions. Myelopathy, however, has not received adequate emphasis as a complication of SLE. Fisher and Gilmour reported the first case of flaccid paraplegia in a female with SLE in 1939. Since then only 25 additional cases have been reported in the medical literature over the past 38 years. Twenty cases have been described in sufficient detail and six briefly, to permit a meaningful review of the spinal cord involvement in this disease. We feel that there are probably many more unreported cases of myelopathy associated with SLE. -

Erythema Marginatum
Figurative Erythemas Michelle Goedken, DO Affiliated Dermatology Scottsdale, AZ Figurative Erythemas • Erythema annulare centrifugum • Erythema marginatum • Erythema migrans • Erythema gyratum repens • Erythema multiforme Erythemas • Erythemas represent a change in the color of the skin that is due to the dilation of blood vessels, especially those in the papillary and reticular dermis • The color is blanchable and most last for days to months • Figurative erythemas have an annular, arciform or polycyclic appearance ERYTHEMA ANNULARE CENTRIFUGUM ERYTHEMA ANNULARE CENTRIFUGUM • Pathogenesis: EAC represents a reaction pattern or hypersensitivity to one of many antigens – IL-2 and TNF-alpha may have a role – Most patients do not have an underlying disease identified ERYTHEMA ANNULARE CENTRIFUGUM • Associated with: – Infection » Dermatophytes and other fungi (Candida and Penicillium in blue cheese) » Viruses: poxvirus, EBV, VZV, HIV » Parasites and ectoparasites – Drugs: diuretics, antimalarials, gold, NSAIDs, finasteride, amitriptyline, etizolam, Ustekinumab (2012) ERYTHEMA ANNULARE CENTRIFUGUM – Foods – Autoimmune endocrinopathies – Neoplasms (lymphomas and leukemias) – Pregnancy – Hypereosinophilic syndrome – Lupus (2014) ERYTHEMA ANNULARE CENTRIFUGUM http://www.dermaamin.com Rongioletti, F., Fausti, V., & Parodi, A ERYTHEMA ANNULARE CENTRIFUGUM • 2 major forms: – Superficial: classic trailing scale, may have associated pruritus – Deep: infiltrated borders, usually no scale, edges are elevated, usually not pruritic ERYTHEMA ANNULARE CENTRIFUGUM -

Diagnosis, Classification, and Management of Erythema
Arch Dis Child 2000;83:347–352 347 Diagnosis, classification, and management of Arch Dis Child: first published as 10.1136/adc.83.4.347 on 1 October 2000. Downloaded from erythema multiforme and Stevens–Johnson syndrome C Léauté-Labrèze, T Lamireau, D Chawki, J Maleville, A Taïeb Abstract become widely accepted that EM and SJS, as Background—In adults, erythema multi- well as toxic epidermal necrolysis, are all part of forme (EM) is thought to be mainly a single “EM spectrum”. In both EM and SJS, related to herpes infection and Stevens– pathological changes in the earliest skin lesion Johnson syndrome (SJS) to drug reac- consist of the accumulation of mononuclear tions. cells around the superficial dermal blood Aims—To investigate this hypothesis in vessels; epidermal damage is more characteris- children, and to review our experience in tic of EM with keratinocyte necrosis leading to the management of these patients. multilocular intraepidermal blisters.5 In fact, Methods—A retrospective analysis of 77 there is little clinical resemblance between paediatric cases of EM or SJS admitted to typical EM and SJS, and recently some authors the Children’s Hospital in Bordeaux be- have proposed a reconsideration of the “spec- tween 1974 and 1998. trum” concept and a return to the original Results—Thirty five cases, inadequately description.15–17 According to these authors, the documented or misdiagnosed mostly as term EM should be restricted to acrally urticarias or non-EM drug reactions were distributed typical targets or raised oedema- excluded. Among the remaining 42 pa- tous papules. Depending on the presence or tients (14 girls and 28 boys), 22 had EM (11 absence of mucous membrane erosions the EM minor and 11 EM major), 17 had SJS, cases may be classified as EM major or EM 16 and three had isolated mucous membrane minor. -

Comorbidities in Psoriasis: the Recognition of Psoriasis As a Systemic Disease and Current Management
Review Derleme 71 DOI: 10.4274/turkderm.09476 Turkderm-Turk Arch Dermatol Venereology 2017;51:71-7 Comorbidities in psoriasis: The recognition of psoriasis as a systemic disease and current management Psoriazisde komorbiditeler: Psoriazisin sistemik hastalık olarak kabul edilmesi ve güncel yaklaşım Göknur Kalkan Ankara Yıldırım Beyazıt University Faculty of Medicine, Department of Dermatology, Ankara, Turkey Abstract Psoriasis, with a worldwide prevalence of 2-3%, is now assumed as a systemic chronic inflammatory disease accompanied by comorbidities while it was accepted as a disease limited only to the skin in the past. There are several classifications of the comorbidities which are more common in patients with moderate to severe psoriasis. Simply, comorbidities can be classified as classic, emerging, related to lifestyle, related to treatment. They can also be categorized as medical comorbidities, psychiatric/psychologic comorbidities, and behaviors contributing to medical and psychiatric comorbidities. In this review, providing early diagnosis and treatment of comorbidities, learning screening recommendations for early detection and long-term disease control and improvement in life quality by integrated, multidisciplinary approach were targeted. Keywords: Psoriasis, comorbidity, systemic disease Öz Tüm dünyada %2-3 oranında görülme sıklığına sahip psoriazis, geçmişte sadece deriye sınırlı kabul edilirken, günümüzde birçok komorbiditenin eşlik ettiği kronik sistemik enflamatuvar bir hastalık olarak ele alınmaktadır. Orta-şiddetli düzeyde -

Fundamentals of Dermatology Describing Rashes and Lesions
Dermatology for the Non-Dermatologist May 30 – June 3, 2018 - 1 - Fundamentals of Dermatology Describing Rashes and Lesions History remains ESSENTIAL to establish diagnosis – duration, treatments, prior history of skin conditions, drug use, systemic illness, etc., etc. Historical characteristics of lesions and rashes are also key elements of the description. Painful vs. painless? Pruritic? Burning sensation? Key descriptive elements – 1- definition and morphology of the lesion, 2- location and the extent of the disease. DEFINITIONS: Atrophy: Thinning of the epidermis and/or dermis causing a shiny appearance or fine wrinkling and/or depression of the skin (common causes: steroids, sudden weight gain, “stretch marks”) Bulla: Circumscribed superficial collection of fluid below or within the epidermis > 5mm (if <5mm vesicle), may be formed by the coalescence of vesicles (blister) Burrow: A linear, “threadlike” elevation of the skin, typically a few millimeters long. (scabies) Comedo: A plugged sebaceous follicle, such as closed (whitehead) & open comedones (blackhead) in acne Crust: Dried residue of serum, blood or pus (scab) Cyst: A circumscribed, usually slightly compressible, round, walled lesion, below the epidermis, may be filled with fluid or semi-solid material (sebaceous cyst, cystic acne) Dermatitis: nonspecific term for inflammation of the skin (many possible causes); may be a specific condition, e.g. atopic dermatitis Eczema: a generic term for acute or chronic inflammatory conditions of the skin. Typically appears erythematous, -

Isotretinoin and the Risk of Erythema Multiforme Final SPC and PL
Isotretinoin and the risk of erythema multiforme Final SPC and PL wording agreed by the PhVWP in June 2010 Doc. Ref.: CMDh/PhVWP/021/2010 Rev 1 July 2010 SUMMARY OF PRODUCT CHARACTERISTICS Section 4.4 There have been post-marketing reports of severe skin reactions (e.g. erythema multiforme (EM), Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN)) associated with isotretinoin use. As these events may be difficult to distinguish from other skin reactions that may occur (see section 4.8), patients should be advised of the signs and symptoms and monitored closely for severe skin reactions. If a severe skin reaction is suspected, isotretinoin treatment should be discontinued. Section 4.8 Skin and subcutaneous tissues Cheilitis, dermatitis, dry skin, localised exfoliation, pruritus, rash disorders: erythematous, skin fragility (risk of frictional trauma) Very common (≥ 1/10) Rare (≥ 1/10 000,< 1/1000) Alopecia Acne fulminans, acne aggravated (acne flare), erythema (facial), exanthema, hair disorders, hirsutism, nail dystrophy, paronychia, Very Rare (≤ 1/10 000) photosensitivity reaction, pyogenic granuloma, skin hyperpigmentation, sweating increased, ∗ Erythema multiforme, Stevens-Johnson Syndrome, toxic Unknown epidermal necrolysis. PACKAGE LEAFLET Possible side effects Skin and hair problems Unknown frequency Serious skin rashes (erythema multiforme, Stevens- Johnson syndrome, and toxic epidermal necrolysis), which are potentially life-threatening and require immediate medical attention. These appear initially as circular patches often with central blisters usually on arms and hands or legs and feet, more severe rashes may include blistering of the chest and back. Additional symptoms such as infection of the eye (conjunctivitis) or ulcers of the mouth, throat or nose may occur.