Transcriptomic Characterisation of the Molecular Mechanisms Induced by Rgma During Skeletal Muscle Hyperplasia and Hypertrophy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ENSG Gene Encodes Effector TCR Pathway Costimulation Inhibitory/Exhaustion Synapse/Adhesion Chemokines/Receptors
ENSG Gene Encodes Effector TCR pathway Costimulation Inhibitory/exhaustion Synapse/adhesion Chemokines/receptors ENSG00000111537 IFNG IFNg x ENSG00000109471 IL2 IL-2 x ENSG00000232810 TNF TNFa x ENSG00000271503 CCL5 CCL5 x x ENSG00000139187 KLRG1 Klrg1 x ENSG00000117560 FASLG Fas ligand x ENSG00000121858 TNFSF10 TRAIL x ENSG00000134545 KLRC1 Klrc1 / NKG2A x ENSG00000213809 KLRK1 Klrk1 / NKG2D x ENSG00000188389 PDCD1 PD-1 x x ENSG00000117281 CD160 CD160 x x ENSG00000134460 IL2RA IL-2 receptor x subunit alpha ENSG00000110324 IL10RA IL-10 receptor x subunit alpha ENSG00000115604 IL18R1 IL-18 receptor 1 x ENSG00000115607 IL18RAP IL-18 receptor x accessory protein ENSG00000081985 IL12RB2 IL-12 receptor x beta 2 ENSG00000186810 CXCR3 CXCR3 x x ENSG00000005844 ITGAL CD11a x ENSG00000160255 ITGB2 CD18; Integrin x x beta-2 ENSG00000156886 ITGAD CD11d x ENSG00000140678 ITGAX; CD11c x x Integrin alpha-X ENSG00000115232 ITGA4 CD49d; Integrin x x alpha-4 ENSG00000169896 ITGAM CD11b; Integrin x x alpha-M ENSG00000138378 STAT4 Stat4 x ENSG00000115415 STAT1 Stat1 x ENSG00000170581 STAT2 Stat2 x ENSG00000126561 STAT5a Stat5a x ENSG00000162434 JAK1 Jak1 x ENSG00000100453 GZMB Granzyme B x ENSG00000145649 GZMA Granzyme A x ENSG00000180644 PRF1 Perforin 1 x ENSG00000115523 GNLY Granulysin x ENSG00000100450 GZMH Granzyme H x ENSG00000113088 GZMK Granzyme K x ENSG00000057657 PRDM1 Blimp-1 x ENSG00000073861 TBX21 T-bet x ENSG00000115738 ID2 ID2 x ENSG00000176083 ZNF683 Hobit x ENSG00000137265 IRF4 Interferon x regulatory factor 4 ENSG00000140968 IRF8 Interferon -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

The Capacity of Long-Term in Vitro Proliferation of Acute Myeloid
The Capacity of Long-Term in Vitro Proliferation of Acute Myeloid Leukemia Cells Supported Only by Exogenous Cytokines Is Associated with a Patient Subset with Adverse Outcome Annette K. Brenner, Elise Aasebø, Maria Hernandez-Valladares, Frode Selheim, Frode Berven, Ida-Sofie Grønningsæter, Sushma Bartaula-Brevik and Øystein Bruserud Supplementary Material S2 of S31 Table S1. Detailed information about the 68 AML patients included in the study. # of blasts Viability Proliferation Cytokine Viable cells Change in ID Gender Age Etiology FAB Cytogenetics Mutations CD34 Colonies (109/L) (%) 48 h (cpm) secretion (106) 5 weeks phenotype 1 M 42 de novo 241 M2 normal Flt3 pos 31.0 3848 low 0.24 7 yes 2 M 82 MF 12.4 M2 t(9;22) wt pos 81.6 74,686 low 1.43 969 yes 3 F 49 CML/relapse 149 M2 complex n.d. pos 26.2 3472 low 0.08 n.d. no 4 M 33 de novo 62.0 M2 normal wt pos 67.5 6206 low 0.08 6.5 no 5 M 71 relapse 91.0 M4 normal NPM1 pos 63.5 21,331 low 0.17 n.d. yes 6 M 83 de novo 109 M1 n.d. wt pos 19.1 8764 low 1.65 693 no 7 F 77 MDS 26.4 M1 normal wt pos 89.4 53,799 high 3.43 2746 no 8 M 46 de novo 26.9 M1 normal NPM1 n.d. n.d. 3472 low 1.56 n.d. no 9 M 68 MF 50.8 M4 normal D835 pos 69.4 1640 low 0.08 n.d. -

Serum Albumin OS=Homo Sapiens
Protein Name Cluster of Glial fibrillary acidic protein OS=Homo sapiens GN=GFAP PE=1 SV=1 (P14136) Serum albumin OS=Homo sapiens GN=ALB PE=1 SV=2 Cluster of Isoform 3 of Plectin OS=Homo sapiens GN=PLEC (Q15149-3) Cluster of Hemoglobin subunit beta OS=Homo sapiens GN=HBB PE=1 SV=2 (P68871) Vimentin OS=Homo sapiens GN=VIM PE=1 SV=4 Cluster of Tubulin beta-3 chain OS=Homo sapiens GN=TUBB3 PE=1 SV=2 (Q13509) Cluster of Actin, cytoplasmic 1 OS=Homo sapiens GN=ACTB PE=1 SV=1 (P60709) Cluster of Tubulin alpha-1B chain OS=Homo sapiens GN=TUBA1B PE=1 SV=1 (P68363) Cluster of Isoform 2 of Spectrin alpha chain, non-erythrocytic 1 OS=Homo sapiens GN=SPTAN1 (Q13813-2) Hemoglobin subunit alpha OS=Homo sapiens GN=HBA1 PE=1 SV=2 Cluster of Spectrin beta chain, non-erythrocytic 1 OS=Homo sapiens GN=SPTBN1 PE=1 SV=2 (Q01082) Cluster of Pyruvate kinase isozymes M1/M2 OS=Homo sapiens GN=PKM PE=1 SV=4 (P14618) Glyceraldehyde-3-phosphate dehydrogenase OS=Homo sapiens GN=GAPDH PE=1 SV=3 Clathrin heavy chain 1 OS=Homo sapiens GN=CLTC PE=1 SV=5 Filamin-A OS=Homo sapiens GN=FLNA PE=1 SV=4 Cytoplasmic dynein 1 heavy chain 1 OS=Homo sapiens GN=DYNC1H1 PE=1 SV=5 Cluster of ATPase, Na+/K+ transporting, alpha 2 (+) polypeptide OS=Homo sapiens GN=ATP1A2 PE=3 SV=1 (B1AKY9) Fibrinogen beta chain OS=Homo sapiens GN=FGB PE=1 SV=2 Fibrinogen alpha chain OS=Homo sapiens GN=FGA PE=1 SV=2 Dihydropyrimidinase-related protein 2 OS=Homo sapiens GN=DPYSL2 PE=1 SV=1 Cluster of Alpha-actinin-1 OS=Homo sapiens GN=ACTN1 PE=1 SV=2 (P12814) 60 kDa heat shock protein, mitochondrial OS=Homo -

SUPPLEMENTARY MATERIAL Bone Morphogenetic Protein 4 Promotes
www.intjdevbiol.com doi: 10.1387/ijdb.160040mk SUPPLEMENTARY MATERIAL corresponding to: Bone morphogenetic protein 4 promotes craniofacial neural crest induction from human pluripotent stem cells SUMIYO MIMURA, MIKA SUGA, KAORI OKADA, MASAKI KINEHARA, HIROKI NIKAWA and MIHO K. FURUE* *Address correspondence to: Miho Kusuda Furue. Laboratory of Stem Cell Cultures, National Institutes of Biomedical Innovation, Health and Nutrition, 7-6-8, Saito-Asagi, Ibaraki, Osaka 567-0085, Japan. Tel: 81-72-641-9819. Fax: 81-72-641-9812. E-mail: [email protected] Full text for this paper is available at: http://dx.doi.org/10.1387/ijdb.160040mk TABLE S1 PRIMER LIST FOR QRT-PCR Gene forward reverse AP2α AATTTCTCAACCGACAACATT ATCTGTTTTGTAGCCAGGAGC CDX2 CTGGAGCTGGAGAAGGAGTTTC ATTTTAACCTGCCTCTCAGAGAGC DLX1 AGTTTGCAGTTGCAGGCTTT CCCTGCTTCATCAGCTTCTT FOXD3 CAGCGGTTCGGCGGGAGG TGAGTGAGAGGTTGTGGCGGATG GAPDH CAAAGTTGTCATGGATGACC CCATGGAGAAGGCTGGGG MSX1 GGATCAGACTTCGGAGAGTGAACT GCCTTCCCTTTAACCCTCACA NANOG TGAACCTCAGCTACAAACAG TGGTGGTAGGAAGAGTAAAG OCT4 GACAGGGGGAGGGGAGGAGCTAGG CTTCCCTCCAACCAGTTGCCCCAAA PAX3 TTGCAATGGCCTCTCAC AGGGGAGAGCGCGTAATC PAX6 GTCCATCTTTGCTTGGGAAA TAGCCAGGTTGCGAAGAACT p75 TCATCCCTGTCTATTGCTCCA TGTTCTGCTTGCAGCTGTTC SOX9 AATGGAGCAGCGAAATCAAC CAGAGAGATTTAGCACACTGATC SOX10 GACCAGTACCCGCACCTG CGCTTGTCACTTTCGTTCAG Suppl. Fig. S1. Comparison of the gene expression profiles of the ES cells and the cells induced by NC and NC-B condition. Scatter plots compares the normalized expression of every gene on the array (refer to Table S3). The central line -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -

Role of the Nuclear Receptor Rev-Erb Alpha in Circadian Food Anticipation and Metabolism Julien Delezie
Role of the nuclear receptor Rev-erb alpha in circadian food anticipation and metabolism Julien Delezie To cite this version: Julien Delezie. Role of the nuclear receptor Rev-erb alpha in circadian food anticipation and metabolism. Neurobiology. Université de Strasbourg, 2012. English. NNT : 2012STRAJ018. tel- 00801656 HAL Id: tel-00801656 https://tel.archives-ouvertes.fr/tel-00801656 Submitted on 10 Apr 2013 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. UNIVERSITÉ DE STRASBOURG ÉCOLE DOCTORALE DES SCIENCES DE LA VIE ET DE LA SANTE CNRS UPR 3212 · Institut des Neurosciences Cellulaires et Intégratives THÈSE présentée par : Julien DELEZIE soutenue le : 29 juin 2012 pour obtenir le grade de : Docteur de l’université de Strasbourg Discipline/ Spécialité : Neurosciences Rôle du récepteur nucléaire Rev-erbα dans les mécanismes d’anticipation des repas et le métabolisme THÈSE dirigée par : M CHALLET Etienne Directeur de recherche, université de Strasbourg RAPPORTEURS : M PFRIEGER Frank Directeur de recherche, université de Strasbourg M KALSBEEK Andries -
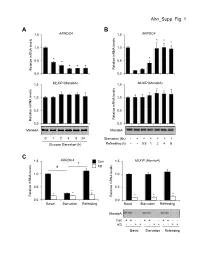
Ahn Supp. Fig. 1 AB 1.5 ARRDC4 1.5 ARRDC4 * * * 1.0 1.0
Ahn_Supp. Fig. 1 AB 1.5 ARRDC4 1.5 ARRDC4 * * * 1.0 1.0 * * 0.5 * 0.5 * * * Relative mRNA levels mRNA Relative Relative mRNA levels mRNA Relative 0.0 0.0 1.5 MLXIP (MondoA) 1.5 MLXIP (MondoA) 1.0 1.0 0.5 0.5 Relative mRNA levels mRNA Relative Relative mRNA levels mRNA Relative 0.0 0.0 MondoA MondoA 0124824 Starvation (6h) -++++++ Glucose Starvation (h) Refeeding (h) --0.51248 C 1.5 ARRDC4 1.5 MLXIP (MondoA) † Con # KD 1.0 1.0 0.5 0.5 * * * * Relative mRNA levels mRNA Relative Relative mRNA levels mRNA Relative * * 0.0 0.0 BasalStarvation Refeeding BasalStarvation Refeeding MondoA Con + + - - + + - - + + - - KD - - + + - - + + - - + + BasalStarvation Refeeding Supplemental Figure 1. Glucose-mediated regulation of ARRDC4 is dependent on MondoA in human skeletal myotubes. (A) (top) ARRDC4 and MLXIP (MondoA) mRNA levels were determined by qRT-PCR in human skeletal myotubes following deprivation of glucose at the indicated time (n=4). (bottom) Representative Western blot analysis of MondoA demonstrating the effect of glucose deprivation. *p<0.05 vs. 0h. (B) (top) ARRDC4 and MLXIP (MondoA) expression in human myotubes following a 6h glucose removal and refeeding at the times indicated (n=4). (bottom) Corresponding Western blot analysis. *p<0.05 vs Starvation 6h. (C) (top) Expression of ARRDC4 and MLXIP in human myotubes following deprivation and refeeding of glucose in the absence or presence of siRNA-mediated MondoA KD (n=4). (bottom) Corresponding Western blot analysis. *p<0.05 vs siControl. # p<0.05. § p<0.05. The data represents mean ± SD. All statistical significance determined by one-way ANOVA with Tukey multiple comparison post-hoc test. -

Temporal Change in Gene Expression in the Rat Dentate Gyrus Following Passive Avoidance Learning
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by MURAL - Maynooth University Research Archive Library Journal of Neurochemistry, 2007, 101, 1085–1098 doi:10.1111/j.1471-4159.2006.04418.x Temporal change in gene expression in the rat dentate gyrus following passive avoidance learning Niamh C. O’Sullivan,* Paul A. McGettigan,* Graham K. Sheridan,* Mark Pickering,* Lisa Conboy,* John J. O’Connor,* Paul N. Moynagh,* Desmond G. Higgins, Ciaran M. Regan* and Keith J. Murphy* *Applied Neurotherapeutics Research Group, UCD Schools of Biomolecular and Biomedical Science, UCD Conway Institute, University College Dublin, Belfield, Dublin, Ireland Applied Neurotherapeutics Research Group, UCD Schools of Medicine and Medical Science, UCD Conway Institute, University College Dublin, Belfield, Dublin, Ireland Abstract identified the likely transcription factors controlling gene A learning event initiates a cascade of altered gene expression in each post-training period. The role of NFjB, expression leading to synaptic remodelling within the hippo- implicated in the early post-training period was subsequently campal dentate gyrus, a structure vital to memory formation. confirmed with activation and nuclear translocation seen in To illuminate this transcriptional program of synaptic plasticity dentate granule neurons following training. mRNA changes we used microarrays to quantify mRNA from the rat dentate for four genes, LRP3 (0 h), alpha actin (3 h), SNAP25 and gyrus at increasing times following passive avoidance NSF (6–12 h), were validated at message and/or protein learning. Approximately, 500 known genes were transcrip- level and shown to be learning specific. Thus, the memory- tionally regulated across the 24 h post-training period. -

The Master Regulator Gene PRDM2 Controls C2C12 Myoblasts
Open Access Insights in Biology and Medicine Research Article The master regulator gene PRDM2 controls C2C12 myoblasts proliferation ISSN 2639-6769 and Differentiation switch and PRDM4 and PRDM10 expression Di Zazzo Erika1#, Bartollino Silvia1*# and Moncharmont Bruno1 1Department of Medicine and Health Sciences “V. Tiberio”, University of Molise, Italy #These authors contributed equally to this work *Address for Correspondence: Silvia Bartollino, Abstract Department of Medicine and Health Sciences, “V. Tiberio”, University of Molise Via F. De Sanctis, The Positive Regulatory Domain (PRDM) protein family gene is involved in a spectrum variety of biological 86100 Campobasso, Italy, Tel.: +39 0874404886, processes, including proliferation, differentiation and apoptosis: its member seem to be transcriptional E-mail: [email protected] regulators highly cell type and tissue peculiar, towards histones modifi cations or recruitment of specifi c Submitted: 11 August 2017 interaction patters to modify the expression of target genes. In this study we analyzed the expression profi le of Approved: 20 September 2017 different member of PRDM gene family focusing our attention on the role of PRDM2, PRDM4 and PRDM10 genes Published: 25 September 2017 in mouse C2C12 cell line, during the differentiation of myoblasts into myotubes and speculate about the role of the protein Retinoblastoma protein-interacting zinc fi nger protein 1-RIZ1, coded by PRDM2 gene, as a regulator Copyright: 2017 Di Zazzo E, et al. This is an open access article distributed under the of the proliferation/differentiation switch. Creative Commons Attribution License, which permits unrestricted use, distribution, and Results showed a reduction of PRDM2, PRDM4 and PRDM10 expression level during the commitment of the reproduction in any medium, provided the differentiation of myoblasts into myotubes. -

Dietary Genistein Supplementation for Breeders and Their Offspring
www.nature.com/scientificreports OPEN Dietary genistein supplementation for breeders and their ofspring improves the growth performance Received: 29 November 2017 Accepted: 13 March 2018 and immune function of broilers Published: xx xx xxxx Zengpeng Lv, Hao Fan, Beibei Zhang, Kun Xing & Yuming Guo Genistein (GEN) is mainly extracted from soy plants and has potential functions as an antioxidant and in promoting immune function and growth. This study evaluated the efects of feeding breeders and their ofspring dietary GEN on the immune function and growth performance of broiler chicks. Breeders were assigned to a control diet or GEN diet (control diet +400 mg/kg GEN), and their ofspring were fed a control diet or GEN diet (control diet +40 mg/kg GEN). GEN treatment increased the body weight gain, tibial length, tibial width and slaughter performance of broilers and decreased the feed conversion ratio. The treatment also afected skeletal muscle myosin assembly and growth and increased growth hormone levels and IGF-I and IGFBP1 expression. Following GEN treatment, antigen processing and presentation, macrophage activation, B lymphocyte, NK cell and helper T cell proliferation, and CD4+ T lymphocyte diferentiation all increased signifcantly. Increases were also observed in IgM and IgG concentrations, antibody titers, and antioxidant capacity. In addition, GEN treatment activated the Toll-like receptor signaling pathway and MAPK cascade signaling pathway. In summary, dietary GEN supplementation for breeders and their ofspring can improve the growth performance and immune function of broiler chicks. Isofavones (ISFs), including genistein (GEN), daidzein and glycitein, are widely found in soy plants and have potential functions as antioxidants and in immune function and detoxifcation1. -

Potential Applications for Mrna and Peptide-Based Vaccines
viruses Article An Epitope Platform for Safe and Effective HTLV-1-Immunization: Potential Applications for mRNA and Peptide-Based Vaccines Guglielmo Lucchese 1,*,†, Hamid Reza Jahantigh 2,3,† , Leonarda De Benedictis 2, Piero Lovreglio 2 and Angela Stufano 2,3 1 Department of Neurology, Medical University of Greifswald, 17475 Greifswald, Germany 2 Interdisciplinary Department of Medicine-Section of Occupational Medicine, University of Bari, 70124 Bari, Italy; [email protected] (H.R.J.); [email protected] (L.D.B.); [email protected] (P.L.); [email protected] (A.S.) 3 Animal Health and Zoonosis Doctoral Program, Department of Veterinary Medicine, University of Bari, 70010 Bari, Italy * Correspondence: [email protected] † These authors contributed equally to the work. Abstract: Human T-cell lymphotropic virus type 1 (HTLV-1) infection affects millions of individuals worldwide and can lead to severe leukemia, myelopathy/tropical spastic paraparesis, and numerous other disorders. Pursuing a safe and effective immunotherapeutic approach, we compared the viral polyprotein and the human proteome with a sliding window approach in order to identify oligopeptide sequences unique to the virus. The immunological relevance of the viral unique oligopeptides was assessed by searching them in the immune epitope database (IEDB). We found that Citation: Lucchese, G.; Jahantigh, HTLV-1 has 15 peptide stretches each consisting of uniquely viral non-human pentapeptides which H.R.; De Benedictis, L.; Lovreglio, P.; Stufano, A. An Epitope Platform for are ideal candidate for a safe and effective anti-HTLV-1 vaccine. Indeed, experimentally validated Safe and Effective HTLV-1 epitopes, as retrieved from the IEDB, contain peptide sequences also present in a vast number HTLV-1-Immunization: Potential of human proteins, thus potentially instituting the basis for cross-reactions.