A Study of the Fowl River Sub Watershed
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mobile 1 Cemetery Locale Location Church Affiliation and Remarks
Mobile 1 Cemetery Locale Location Church Affiliation and Remarks Ahavas Chesed Inset - 101 T4S, R1W, Sec 27 adjacent to Jewish Cemetery; approximately 550 graves; Berger, Berman, Berson, Brook, Einstein, Friedman, Frisch, Gernhardt, Golomb, Gotlieb, Gurwitch, Grodsky, Gurwitch, Haiman, Jaet, Kahn, Lederman, Liebeskind, Loeb, Lubel, Maisel, Miller, Mitchell, Olensky, Plotka, Rattner, Redisch, Ripps, Rosner, Schwartz, Sheridan, Weber, Weinstein and Zuckerman are common to this active cemetery (35) All Saints Inset - 180 T4S, R1W, Sec 27 All Saints Episcopal Church; 22 graves; first known interment: Louise Shields Ritter (1971-1972); Bond and Ritter are the only surnames of which there are more than one interment in this active cemetery (35) Allentown 52 - NW T3S, R3W, Sec 29 established 1850, approximately 550 graves; first known interment: Nancy Howell (1837-1849); Allen, Busby, Clark, Croomes, Ernest, Fortner, Hardeman, Howell, Hubbard, Jordan, Lee, Lowery, McClure, McDuffie, Murphree, Pierce, Snow, Tanner, Waltman and Williams are common to this active cemetery (8) (31) (35) Alvarez Inset - 67 T2S, R1W, Sec 33 see Bailey Andrus 151 - NE T2S, R1W, Sec 33 located on Graham Street off Celest Road in Saraland, also known as Saraland or Strange; the graves of Lizzie A. Macklin Andrus (1848-1906), Alicia S. Lathes Andrus (1852-1911) and Pelunia R. Poitevent Andrus (1866-1917), all wives of T. W. Andrus (1846-1925) (14) (35) Axis 34 - NE T1S, R1E, Sec 30 also known as Bluff Cemetery; 12 marked and 9 unmarked graves; first interment in 1905; last known interment: Willie C. Williams (1924-1991); Ames, Ethel, Green, Hickman, Lewis, Rodgers and Williams are found in this neglected cemetery (14) (31) (35) Bailey Inset - 67 T2S, R1W, Sec 33 began as Alvarez Cemetery, also known as Saraland Cemetery; a black cemetery of approximately 325 marked and 85 unmarked graves; first known interment: Emmanuel Alvarez (d. -
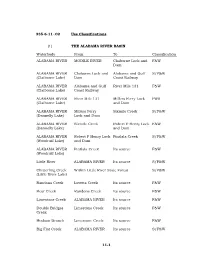
11-1 335-6-11-.02 Use Classifications. (1) the ALABAMA RIVER BASIN Waterbody from to Classification ALABAMA RIVER MOBILE RIVER C
335-6-11-.02 Use Classifications. (1) THE ALABAMA RIVER BASIN Waterbody From To Classification ALABAMA RIVER MOBILE RIVER Claiborne Lock and F&W Dam ALABAMA RIVER Claiborne Lock and Alabama and Gulf S/F&W (Claiborne Lake) Dam Coast Railway ALABAMA RIVER Alabama and Gulf River Mile 131 F&W (Claiborne Lake) Coast Railway ALABAMA RIVER River Mile 131 Millers Ferry Lock PWS (Claiborne Lake) and Dam ALABAMA RIVER Millers Ferry Sixmile Creek S/F&W (Dannelly Lake) Lock and Dam ALABAMA RIVER Sixmile Creek Robert F Henry Lock F&W (Dannelly Lake) and Dam ALABAMA RIVER Robert F Henry Lock Pintlala Creek S/F&W (Woodruff Lake) and Dam ALABAMA RIVER Pintlala Creek Its source F&W (Woodruff Lake) Little River ALABAMA RIVER Its source S/F&W Chitterling Creek Within Little River State Forest S/F&W (Little River Lake) Randons Creek Lovetts Creek Its source F&W Bear Creek Randons Creek Its source F&W Limestone Creek ALABAMA RIVER Its source F&W Double Bridges Limestone Creek Its source F&W Creek Hudson Branch Limestone Creek Its source F&W Big Flat Creek ALABAMA RIVER Its source S/F&W 11-1 Waterbody From To Classification Pursley Creek Claiborne Lake Its source F&W Beaver Creek ALABAMA RIVER Extent of reservoir F&W (Claiborne Lake) Beaver Creek Claiborne Lake Its source F&W Cub Creek Beaver Creek Its source F&W Turkey Creek Beaver Creek Its source F&W Rockwest Creek Claiborne Lake Its source F&W Pine Barren Creek Dannelly Lake Its source S/F&W Chilatchee Creek Dannelly Lake Its source S/F&W Bogue Chitto Creek Dannelly Lake Its source F&W Sand Creek Bogue -

1Ba704, a NINETEENTH CENTURY SHIPWRECK SITE in the MOBILE RIVER BALDWIN and MOBILE COUNTIES, ALABAMA
ARCHAEOLOGICAL INVESTIGATIONS OF 1Ba704, A NINETEENTH CENTURY SHIPWRECK SITE IN THE MOBILE RIVER BALDWIN AND MOBILE COUNTIES, ALABAMA FINAL REPORT PREPARED FOR THE ALABAMA HISTORICAL COMMISSION, THE PEOPLE OF AFRICATOWN, NATIONAL GEOGRAPHIC SOCIETY AND THE SLAVE WRECKS PROJECT PREPARED BY SEARCH INC. MAY 2019 ARCHAEOLOGICAL INVESTIGATIONS OF 1Ba704, A NINETEENTH CENTURY SHIPWRECK SITE IN THE MOBILE RIVER BALDWIN AND MOBILE COUNTIES, ALABAMA FINAL REPORT PREPARED FOR THE ALABAMA HISTORICAL COMMISSION 468 SOUTH PERRY STREET PO BOX 300900 MONTGOMERY, ALABAMA 36130 PREPARED BY ______________________________ JAMES P. DELGADO, PHD, RPA SEARCH PRINCIPAL INVESTIGATOR WITH CONTRIBUTIONS BY DEBORAH E. MARX, MA, RPA KYLE LENT, MA, RPA JOSEPH GRINNAN, MA, RPA ALEXANDER J. DECARO, MA, RPA SEARCH INC. WWW.SEARCHINC.COM MAY 2019 SEARCH May 2019 Archaeological Investigations of 1Ba704, A Nineteenth-Century Shipwreck Site in the Mobile River Final Report EXECUTIVE SUMMARY Between December 12 and 15, 2018, and on January 28, 2019, a SEARCH Inc. (SEARCH) team of archaeologists composed of Joseph Grinnan, MA, Kyle Lent, MA, Deborah Marx, MA, Alexander DeCaro, MA, and Raymond Tubby, MA, and directed by James P. Delgado, PhD, examined and documented 1Ba704, a submerged cultural resource in a section of the Mobile River, in Baldwin County, Alabama. The team conducted current investigation at the request of and under the supervision of Alabama Historical Commission (AHC); Alabama State Archaeologist, Stacye Hathorn of AHC monitored the project. This work builds upon two earlier field projects. The first, in March 2018, assessed the Twelvemile Wreck Site (1Ba694), and the second, in July 2018, was a comprehensive remote-sensing survey and subsequent diver investigations of the east channel of a portion the Mobile River (Delgado et al. -

Guide to the Clarence L. Hutchisson Jr. Papers
Guide to the Clarence L. Hutchisson Jr. Papers Descriptive Summary: Creator: Clarence L. Hutchisson Jr., 1902-1993 Title: Clarence L. Hutchisson Jr. Papers Dates: 1856-1956 (bulk 1927-1956) Quantity: 81.2 linear feet Abstract: Blueprints, correspondence, drawings, etching plates, news clippings, and a scrapbook related to the business dealings and genealogy of architect Clarence L. Hutchisson Jr. Accession: 10-09-267 ; 267-1993 Biographical Note: Clarence L. Hutchisson Jr., the last of the locally celebrated Hutchisson architects, was born in 1902 in Mobile, Alabama. From 1926 to 1932 Hutchisson worked in the office of his father, Clarence L. Hutchisson Sr. Between 1940 and 1945, Hutchisson trained as an engineer and would serve as chief architect for the Mobile Corps of Engineers. During his career, he designed a variety of structures in the Mobile area. Like his mother, Henrietta Homer Hutchisson, he was interested in the genealogy of the Homer family and he and his mother gathered information about several of his bloodlines. Much of this genealogical correspondence took place with his cousin Annie Homer Wilson and pertains to the Homer family in Nova Scotia, Canada. Hutchisson died in December 1993. Scope and Contents: This collection contains etching plates, news clippings, a scrapbook, and the business stamp of Clarence L. Hutchisson Jr. In addition, the collection is made up of a wide selection of correspondence, both business and private, contracts, building specifications, blueprints, and other related architectural documents. Of particular importance are the 200 architectural drawings of structures designed by the Hutchissons (ca. 1908-1972). These drawings are indexed by address as well as the client's name. -

130868257991690000 Lagniap
2 | LAGNIAPPE | September 17, 2015 - September 23, 2015 LAGNIAPPE ••••••••••••••••••••••••••• WEEKLY SEPTEMBER 17, 2015 – S EPTEMBER 23, 2015 | www.lagniappemobile.com Ashley Trice BAY BRIEFS Co-publisher/Editor Federal prosecutors have secured an [email protected] 11th guilty plea in a long bid-rigging Rob Holbert scheme based in home foreclosures. Co-publisher/Managing Editor 5 [email protected] COMMENTARY Steve Hall Marketing/Sales Director The Trice “behind closed doors” [email protected] secrets revealed. Gabriel Tynes Assistant Managing Editor 12 [email protected] Dale Liesch BUSINESS Reporter Greer’s is promoting its seventh year [email protected] of participating in the “Apples for Jason Johnson Students” initiative. Reporter 16 [email protected] Eric Mann Reporter CUISINE [email protected] A highly anticipated Kevin Lee CONTENTS visit to The Melting Associate Editor/Arts Editor Pot in Mobile proved [email protected] disappointing with Andy MacDonald Cuisine Editor lackluster service and [email protected] forgettable flavors. Stephen Centanni Music Editor [email protected] J. Mark Bryant Sports Writer 18 [email protected] 18 Stephanie Poe Copy Editor COVER Daniel Anderson Mobilian Frank Bolton Chief Photographer III has organized fellow [email protected] veterans from atomic Laura Rasmussen Art Director test site cleanup www.laurarasmussen.com duties to share their Brooke Mathis experiences and Advertising Sales Executive resulting health issues [email protected] and fight for necessary Beth Williams Advertising Sales Executive treatment. [email protected] 2424 Misty Groh Advertising Sales Executive [email protected] ARTS Kelly Woods The University of South Alabama’s Advertising Sales Executive Archaeology Museum reaches out [email protected] to the curious with 12,000 years of Melissa Schwarz 26 history. -

Anthonby B. Schmidt, a Clerk for Hammel's Department Store; Bernard M
Anthonby B. Schmidt, a clerk for Hammel's Department Store; Bernard M. Schmidt, a tailor and Christina Schmidt who was a telephone operator for C.J. Gayfer. # p.727 He worked as a collector for Gayfer's store and boarded at 413 Eslava Street in 1918.+764 Schock, Clifton U.S. Army Corporal Died in Mrs. R.J. 31 December E.* Vancouver, Schock,106 In 1913 1918- 35th Spruce He was Washington of South Cedar Clifton E. [Clifton Edison Squadron born in Disease - (II) Street Schrock was a He is buried Shock] Signal Corps Mobile p.l07 clerk and in Square 11 25 Sept. In1916 Mrs. boarded of Magnolia Note: His name (II) p. 107 1895. (II) Rachel J. together with Cemetery in is listed on the p. 107 Schrock, Ellen L. the City of World Warl widow of Philip Schrock,a Mobile. (II) Monument in N. Schrock teacher at p. 107 Memorial Park, owned 106 Leinkauf Mobile, South Cedar School; Philip Alabama. Street. With N. Schrock, a her lived Philip foreman and Schrock who Mrs. Rache J. was the Schrock, foreman for widow of the Home Philip N. Industry Iron Schrock who Works and owned the Ellen L. residence at Schrock a 106 South teacher at Cedar Street. Boys' Senior % p. 549 Grammar School. # p. 728 In 1915 Clifton E. Note: In 1918 Schock was a Mrs. Rachel J. stevedore and Schock, widow boarded at of Philip N. 106 South Schrock owned Cedar Street. 106 South @p786 Cedar Street. + p.46 In 1916 Clifton E. Schrock was a stevedore and boarded at 106 South Cedar Street. -

High Water Mark Collection for Hurricane Katrina in Alabama FEMA-1605-DR-AL, Task Orders 414 and 421 April 3, 2006 (Final)
High Water Mark Collection for Hurricane Katrina in Alabama FEMA-1605-DR-AL, Task Orders 414 and 421 April 3, 2006 (Final) FOR PUBLIC RELEASE Hazard Mitigation Technical Assistance Program Contract No. EMW-2000-CO-0247 Task Orders 414 & 421 Hurricane Katrina Rapid Response Alabama High Water Mark Collection FEMA-1605-DR-AL Final Report April 3, 2006 Submitted to: Federal Emergency Management Agency Region IV Atlanta, GA Prepared by: URS Group, Inc. 200 Orchard Ridge Drive Suite 101 Gaithersburg, MD 20878 FOR PUBLIC RELEASE HMTAP Task Orders 414 and 421 Final Report April 3, 2006 Table of Contents Abbreviations and Acronyms ................................................................................................................... iii Glossary of Terms...................................................................................................................................... iv Executive Summary.................................................................................................................................. vii Introduction and Purpose of the Study...........................................................................................vii Methodology ...................................................................................................................................... vii Coastal HWM Observations ............................................................................................................viii 1. Introduction .............................................................................................................................................1 -

Just Cebrian 1. Background
The Dauphin Island Sea Lab and the University of South Alabama Faculty Summary- Just Cebrian 1. Background Date of birth: January 24, 1968 Married: Marian Claramunt. Two children 1.1 Educational History School Major Degree Date U. Barcelona Biology B.A 1990 U. Perpignan Oceanology M.S 1991 U. Politecnica Catalunya Marine Sciences Ph. D. 1996 1.2 Professional History Prior to DISL/USA From - To Position Institution 1996-1998 Post-Doctoral Associate Boston University 1999 Research Assistant Professor Boston University 1.3 Professional History at DISL/USA From - To Position Institution 2000-2005 Senior Marine Scientist I DISL 2006-2010 Senior Marine Scientist II DISL 2010-present Senior Marine Scientist III DISL 2000-2005 Assistant Professor USA 2006-present Associate Professor USA 2011-present Full Professor USA 2001-present Adjunct Professor UA 2. Teaching Activities 1 2.1 Lectures Delivered in Courses Prior to DISL/USA Course Description Semester Topic Ecology of Marine Macrophyte (U. Barcelona) Spring 1994 Seagrasses Secondary Production (U. Barcelona) Spring 1996 Herbivory 2.2 Courses Taught Prior to DISL/USA Course Description Semester Seminar in Marine Ecology (Boston U.)-graduate Spring 1998 Marine Botany (Boston U.)-undergraduate Fall 1998, 1999 Coastal Eutrophication (UNAM-Mexico)-graduate Fall 1998 2.3 Undergraduate Courses Taught at DISL/USA Course Description Semester Marine Botany Summer 2000, 2001, 2002, 2003, 2004- co-taught with Dr. Hugh Macintyre-, 2006, 2007, 2008, 2009, 2010, 2011, 2012, 2013, 2104, 2015 2.4 Graduate -

Guide to the Lambert C. Mims Papers
Guide to the Lambert C. Mims Papers Descriptive Summary: Creator: Lambert C. Mims, 1930-2008 Title: Lambert C. Mims Papers Dates: 1820-2003 (bulk 1965-1989) Quantity: 160.5 linear feet Abstract: Papers agendas, audio tapes, books, campaign material, correspondence, flyers, legal material, magazines, maps, negatives, news clippings, notes, pamphlets, photographs, plaques, reports, slides, speeches, and video tapes. Covers a multitude of local subjects typically found within such political collections. Accession: 06-09-459 ; 459-2006 Biographical Note: Lambert C. Mims was born in 1930 in Uriah, Alabama. He moved to Mobile, Alabama, in 1949 and worked as a salesman before co-founding, a year later, a feed company, and, in 1965, branching out on his own. Lambert Mims was public works commissioner and rotating mayor of Mobile from 1965 to 1985. During Mims' time as mayor/commissioner, the city of Mobile experienced the latter part of the modern civil rights movement, completed the Bayway, and unveiled the George C. Wallace Tunnel. It opened Mobile Greyhound Park and saw the Southern Market/City Hall designated a national historic landmark. It reconstructed and opened Fort Condé and celebrated the nation's bicentennial. It witnessed the devastating destruction of hurricanes Camille and Frederic and saw the first oil well drilled in the bay. It witnessed the completion of the I-65 link across the Mobile-Tensaw Delta and celebrated the opening of the Tennessee-Tombigbee Waterway. When first elected, Mims was the youngest city commissioner in Mobile's history. Upon leaving office, Governor George Wallace appointed Mims as his ambassador to the Alabama Waterways Development Agency, a position he held from 1985 until March 1987, and one in which he promoted the Tennessee-Tombigbee Waterway. -

MIP Amendment Development (Not 8 Mount Vernon Water Treatment Plant 16 Designated on Map)
Alabama’s Multiyear Implementation Plan Appendix Attachment 1 Map of Projects Selected for Inclusion in Alabama’s Multiyear Implementation Plan Attachment 1 Direct Component Projects Included in Draft Multiyear Implementation Plan 8 4 7 5 2 13 14 12 9 13 15 6 3 13 1 11 10 ID PROJECT ID PROJECT Alabama State Port Authority Automotive Logistics/RO-RO 1 Aloe Bay Harbour Town Phases I, II, and III 9 Terminal 2 Historic Africatown Welcome Center 10 Ambassadors of the Environment 3 Redevelop Bayou La Batre City Docks 11 Isle Dauphine Beach and Golf Study 4 Northwest Satsuma Water and Sewer Project 12 Innovating St. Louis Street: Mobile’s Technology Corridor 5 Mobile County Blueway Trail 13 Baldwin County ALDOT Capacity Improvements 6 Water Distribution System Upgrades 14 Mobile Greenway Initiative Baldwin Beach Express I-10 to I-65 Extension ROW 7 15 Working Waterfront and Greenspace Restoration Project Acquisition Planning Assistance - MIP Amendment Development (not 8 Mount Vernon Water Treatment Plant 16 designated on map) Attachment 2 Project Selection Process ProjectSelectionProcessFramework for Backinqueueforfutureconsideration Administrator submits MIP MultiyearImplementationPlan(MIP)Development to Treasury for approval to submit individual project grant applications *Project remains on †Administrator *Administrator prepares Submitter enters Administrator hold for future Administrator Administrator posts reviews and denotes Invite, inform, & Restoration Project Yes determines if Yes all project suggestions for consideration by Yes determines -

Two Day Family Itinerary
THINGS TO DO - ATTRACTIONS We’ll let you in on a little secret... When it comes to things to do, Mobile is rich with all sorts of activities. Abundant attractions, outdoor and water-based adventures, historical and cultural pursuits, sports and more await you. It’s just one of the reasons we think you’ll agree that we’re kind of a big deal on the Gulf Coast. Kick off your visit with a 2-Day Family delicious breakfast downtown , then walk over Itinerary to the Gulf coast exploreum to catch their latest hands-on exhibit or IMAX ® film. Right next door is the history museum of mobile , where you’ll be immersed in over 300 years of history. A few blocks away is the one-of-a-kind mobile carnival museum where you’ll revel in all things Mardi Gras. The uss alabama battleship memorial Park is just a short M O C . drive away and while you’re on ‘the causeway’, be sure z T O to hop on an airboat or kayak to discover the Mobile- H S y Tensaw River delta up close. The next day, tour M - n beautiful bellingrath Gardens and home and take a O S n leisurely ride on the southern belle , continuing on to E D y D M Dauphin Island for a day on the soft, white sand A T © Secretly beaches. You’ll enjoy historic fort Gaines and the me Dauphin island sea lab and estuarium as well as bird Aweso watching, water sports and a laid-back, island attitude. -

Preserving Think You Can Mon Louis Island Start a Business? the Business View AUGUST 2016 1 with YOU on the FRONT LINES the Battle in Every Market Is Unique
Mobile Area Chamber of Commerce AUGUST 2016 the Meet the Chamber Board of Advisors Preserving Think You Can Mon Louis Island Start a Business? the business view AUGUST 2016 1 WITH YOU ON THE FRONT LINES The battle in every market is unique. Ally yourself to a technology leader that knows a truly e ective solution comes from keeping people at the center of technology. Our dedicated Client Account Executives provide an unmatched level of agility and responsiveness as they work in person to fi ne-tune our powerful arsenal of communication solutions for your specifi c business. Four solutions. One goal. A proven way to get there— Personal service. We’re here to help you win. cspire.com/business | 855.277.4732 | [email protected] 2 the business view AUGUST 2016 10623 CSpire CSBS PersonalService 9.25x12.indd 1 6/25/15 1:08 PM WHEN LOSING IS NOT AN OPTION. DON’T SETTLE FOR LESS. For more than 50 years, we have represented businesses in high-stakes litigation on a contingent fee basis. Our client list includes thousands of local, regional, and national businesses ranging in size from small family-owned businesses to multinational corporations. We regularly take on the world’s largest and most well-funded companies – and we win. Learn more about our successes at www.cunninghambounds.com/our-successes. No representation is made that the quality of the legal services to be performed is greater than the quality of legal services performed by other lawyers. These recoveries and testimonials are not an indication of future results.