ESMO 2014 Scientific Meeting Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

List Item Withdrawal Assessment Report for Folcepri
20 March 2014 EMA/CHMP/219148/2014 Committee for Medicinal Products for Human Use (CHMP) Assessment report Folcepri International non-proprietary name: etarfolatide Procedure No.: EMEA/H/C/002570/0000 Note Assessment report as adopted by the CHMP with all information of a commercially confidential nature deleted. This AR reflects the CHMP opinion on 20 March 2014, which originally recommended to approve this medicine. The recommendation was conditional to the results of the on-going confirmatory study EC-FV-06. Before the marketing authorisation was granted by the EC, the results of this study became available and did not support the initial recommendation. Subsequently, the company decided to withdraw the application and not to pursue any longer the authorisation for marketing this product. The current report does not include the latest results of this study as the withdrawal of the application did not allow for the CHMP to revise its opinion in light of the new data. For further information please refer to the Q&A which followed the company’s withdrawal of the application. 30 Churchill Place ● Canary Wharf ● London E14 5EU ● United Kingdom Telephone +44 (0)20 3660 6000 Facsimile +44 (0)20 3660 5505 Send a question via our website www.ema.europa.eu/contact An agency of the European Union © European Medicines Agency, 2014. Reproduction is authorised provided the source is acknowledged. Table of contents 1. Background information on the procedure .............................................. 6 1.1. Submission of the dossier ...................................................................................... 6 1.2. Manufacturers ...................................................................................................... 8 1.3. Steps taken for the assessment of the product ......................................................... 8 2. Scientific discussion ............................................................................... -

545 © American Association of Pharmaceutical Scientists 2019 P. V
Index A Although, 524 Aberrant expression, 118 Alzheimer’s disease, 305, 326 Abluminal, 47 American type culture collection (ATCC), 537 Actin, 7 AMH, see Anti-Mullerian hormone (AMH) Actinic keratosis, 342 Aminolevulinic acid, 501 Actinomycetes, 485 Amino-triphenyl dicarboxylate-bridged Zr4+ Activated macrophages, 13 metal-organic framework nanoparticles Activation, 93 (NMOFs), 215 Activation functions (AFs), 89 Amphiregulin (AREG), 240 Active targeting, 467 β-Amyloid fibrils, 305 Adamantane–hyaluronic acid, 420 Anaplastic large cell lymphoma (ALCL), 219 Adamantane polyethylene glycol, 473 Anaplastic lymphoma kinase (ALK), 233 Adaptive immune responses, 328 Ancillary targets, 165 Adaptor proteins, 15 Androgen receptor (AR), 115 Adenocarcinoma, 231 Androgen receptor antagonists, 123 Adenomatous polyposis coli (APC), 188, 191 Androgen response elements (ARE), 116 Adenosine triphosphate (ATP), 243 Androgens, 120 Adherens junction, 179 Ang2 inhibitor (recombinant peptide-Fc- Adsorptive endocytosis, 49 fusion protein), 215 Advanced chemorefractory endometrial Angiogenesis, 53, 490, 529 cancers, 193 Angiogenesis factors, 189 Advanced epithelial ovarian, 131 Angiogenic paracrine factors, 490 Advanced gastric adenocarcinoma, 220 Anilinoquinazoline tyrosine kinase inhibitor, 242 Advanced glycation end products (AGE), 305 Annexin V, 493 Advanced/metastatic NSCLC, 258 Annexin V-FITC/propidium iodide assay, Adverse effects, 400 534–536 A glycoprotein hormone, 121 Antagonists, 287, 393 Agonists, 87 Antiangiogenic activity, 527 AIDS, 280 Antiangiogenic -

Esmo – Missed Target Leaves Endocyte Clutching at Straws
September 28, 2014 Esmo – Missed Target leaves Endocyte clutching at straws Jacob Plieth Followers of Endocyte had pinned their hopes on overall survival data from vintafolide’s phase II Target study providing definitive proof of efficacy. With results presented at the Esmo congress on Saturday, however, defeat has been snatched from the jaws of victory. Even a broad data dredge has barely managed to tease out a subgroup benefit – after one accepts Target’s advantageous statistical design, that is. Endocyte had risen 6% on Thursday in anticipation of the late-breaking Esmo data, but when the markets open again tomorrow investors will almost certainly be left nursing losses. There were already question marks around vintafolide, Endocyte’s lead project. European approval was revoked after the Proceed study in ovarian cancer was halted for futility, prompting Merck & Co, which had paid $120m up front to access vintafolide, to pull out of the deal (Trial miss leaves Endocyte at the mercy of Merck, May 6, 2014). Back in March, progression-free survival (PFS) results from the Target trial, which pitched vintafolide and docetaxel against docetaxel alone in a second-line setting for NSCLC, caused Endocyte to surge, and the company managed to raise $102m from investors. The fate of vintafolide, which comprises vinblastine linked to a compound that targets the folate receptor, thus rested on further analyses of Target – most crucially of patients’ overall survival (OS). In fact, the OS data showed vintafolide scoring a miss. Risk of death was reduced by 12% – though with the upper confidence interval bound at 1.36 – with a p value of 0.2874. -

(Ec145) and Pegylated Liposomal Doxorubicin (Pld/Doxil®/Caelyx®) in Combination Versus Pld in Patients with Platinum-Resistant Ovarian Cancer
EC-FV-06 Clinical Study Report Page 1 1. EC-FV-06 Clinical Study Report A RANDOMIZED DOUBLE-BLIND PHASE 3 TRIAL COMPARING VINTAFOLIDE (EC145) AND PEGYLATED LIPOSOMAL DOXORUBICIN (PLD/DOXIL®/CAELYX®) IN COMBINATION VERSUS PLD IN PATIENTS WITH PLATINUM-RESISTANT OVARIAN CANCER Vintafolide (EC145): Targeted Therapeutic Agent 99mTc-etarfolatide: Companion Diagnostic Imaging Agent Therapy for platinum-resistant epithelial ovarian, fallopian tube or primary peritoneal cancer This was an international, multicenter, centrally-randomized, double-blind, Phase 3, two-arm study comparing EC145 + PLD and placebo + PLD, given until disease progression or unacceptable toxicity in patients platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer Endocyte, Inc. Protocol EC-FV-06 (PROCEED) Phase 3 First patient enrolled (assigned to therapy): 22 April 2011 Date of early study termination: 20 May 2014 Data cutoff date: 17 March 2014 Approval Date: 15 February 2017 Responsible Medical Officer: Alison Armour MB.ChB., BSc., MSc., MD., MRCP., FRCR Endocyte, Inc. 8910 Purdue Road, Suite 250 Indianapolis, IN 46268 This study was performed in compliance with the principles of good clinical practice (GCP) and Endocyte, Inc. standard operating procedures. The information contained in this clinical study report is confidential and may not be reproduced or otherwise disseminated without the written approval of Endocyte, Inc.. This document and its associated appendices are subject to United States Freedom of Information Act (FOIA) Exemption 4. Vintafolide (EC145) EC-FV-06 Clinical Study Report Page 2 2. Synopsis Title of Study: A Randomized Double-Blind Phase 3 Trial Comparing Vintafolide (EC145) and Pegylated Liposomal Doxorubicin (PLD/DOXIL®/CAELYX®) in Combination Versus PLD in Patients with Platinum- Resistant Ovarian Cancer Number of Investigator(s): This multicenter study included 194 principal investigators. -

Access to Cancer Medicines in Australia
Access to cancer medicines in Australia Medicines Australia Oncology Industry Taskforce July 2013 Contents Glossary ..................................................................................................................................... i Executive summary .................................................................................................................... i 1 Background ..................................................................................................................... 1 1.1 Purpose of this report ....................................................................................................... 2 1.2 Methods ........................................................................................................................... 3 1.3 Report structure ............................................................................................................... 9 2 Cancer in Australia and other countries ......................................................................... 10 2.1 Population statistics on cancer ........................................................................................ 10 2.2 Population impacts of cancer in Australia ........................................................................ 24 2.3 Summary ........................................................................................................................ 33 3 Current and future cancer medicines ............................................................................ 34 3.1 Current -

Annexes to the Annual Report of the European Medicines Agency 2014
Annexes to the annual report of the European Medicines Agency 2014 Table of contents Annex 1 – Members of the Management Board ............................................................................. 2 Annex 2 – Members of the Committee for Medicinal Products for Human Use ................................... 4 Annex 3 – Members of the Pharmacovigilance Risk Assessment Committee ...................................... 6 Annex 4 – Members of the Committee for Medicinal Products for Veterinary Use ............................... 8 Annex 5 – Members of the Committee on Orphan Medicinal Products ............................................ 10 Annex 6 – Members of the Committee on Herbal Medicinal Products .............................................. 12 Annex 07 – Committee for Advanced Therapies .......................................................................... 14 Annex 8 – Members of the Paediatric Committee ........................................................................ 16 Annex 9 – Working parties and working groups .......................................................................... 18 Annex 10 – CHMP opinions in 2014 on medicinal products for human use ...................................... 22 Annex 11 – CVMP opinions in 2014 on medicinal products for veterinary use .................................. 36 Annex 12 – COMP opinions in 2014 on designation of orphan medicinal products ............................ 41 Annex 13 – HMPC European Union herbal monographs in 2014.................................................... -

Rational Combination Therapy of Vintafolide (EC145) with Commonly Used Chemotherapeutic Drugs
Author Manuscript Published OnlineFirst on January 15, 2014; DOI: 10.1158/1078-0432.CCR-13-2423 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. 1 Rational combination therapy of vintafolide (EC145) with commonly used chemotherapeutic drugs Joseph A. Reddy1, Ryan Dorton1, Alicia Bloomfield1, Melissa Nelson1, Marilynn Vetzel1, John Guan1 and Christopher P. Leamon1 Endocyte, Inc., 3000 Kent Ave, Suite A1-100, West Lafayette, IN 47906, USA 1Corresponding author: Dr. Christopher P. Leamon 3000 Kent Ave. Suite A1-100 West Lafayette, IN 47906 Phone: (765)463-7175 FAX: (765)463-9271 Email: [email protected] Running Title: Vintafolide drug combination studies Keywords: Folate receptor, targeted chemotherapy, ovarian cancer, EC145, vintafolide Downloaded from clincancerres.aacrjournals.org on September 30, 2021. © 2014 American Association for Cancer Research. Author Manuscript Published OnlineFirst on January 15, 2014; DOI: 10.1158/1078-0432.CCR-13-2423 Author manuscripts have been peer reviewed and accepted for publication but have not yet been edited. 2 TRANSLATIONAL RELEVANCE Our report highlights the potential for safely combining vintafolide (a folate-targeted vinca alkaloid) with pegylated liposomal doxorubicin (PLD), platinum-based agents, topoisomerase inhibitors and taxanes to yield enhanced antitumor effects, including complete responses and cures against human tumor xenografts. The observed therapeutic benefits of these combinations were specific for folate receptor (FR)-positive tumor models, likely because vintafolide remains associated with tumor cells for multiple M phases of the cell due to its natural high affinity binding to the FR and the slow recycling rate of that receptor. Based on the disclosed preclinical data, a randomized phase 2 trial (PRECEDENT) was conducted with the vintafolide/PLD combination. -

Patent Application Publication ( 10 ) Pub . No . : US 2019 / 0192440 A1
US 20190192440A1 (19 ) United States (12 ) Patent Application Publication ( 10) Pub . No. : US 2019 /0192440 A1 LI (43 ) Pub . Date : Jun . 27 , 2019 ( 54 ) ORAL DRUG DOSAGE FORM COMPRISING Publication Classification DRUG IN THE FORM OF NANOPARTICLES (51 ) Int . CI. A61K 9 / 20 (2006 .01 ) ( 71 ) Applicant: Triastek , Inc. , Nanjing ( CN ) A61K 9 /00 ( 2006 . 01) A61K 31/ 192 ( 2006 .01 ) (72 ) Inventor : Xiaoling LI , Dublin , CA (US ) A61K 9 / 24 ( 2006 .01 ) ( 52 ) U . S . CI. ( 21 ) Appl. No. : 16 /289 ,499 CPC . .. .. A61K 9 /2031 (2013 . 01 ) ; A61K 9 /0065 ( 22 ) Filed : Feb . 28 , 2019 (2013 .01 ) ; A61K 9 / 209 ( 2013 .01 ) ; A61K 9 /2027 ( 2013 .01 ) ; A61K 31/ 192 ( 2013. 01 ) ; Related U . S . Application Data A61K 9 /2072 ( 2013 .01 ) (63 ) Continuation of application No. 16 /028 ,305 , filed on Jul. 5 , 2018 , now Pat . No . 10 , 258 ,575 , which is a (57 ) ABSTRACT continuation of application No . 15 / 173 ,596 , filed on The present disclosure provides a stable solid pharmaceuti Jun . 3 , 2016 . cal dosage form for oral administration . The dosage form (60 ) Provisional application No . 62 /313 ,092 , filed on Mar. includes a substrate that forms at least one compartment and 24 , 2016 , provisional application No . 62 / 296 , 087 , a drug content loaded into the compartment. The dosage filed on Feb . 17 , 2016 , provisional application No . form is so designed that the active pharmaceutical ingredient 62 / 170, 645 , filed on Jun . 3 , 2015 . of the drug content is released in a controlled manner. Patent Application Publication Jun . 27 , 2019 Sheet 1 of 20 US 2019 /0192440 A1 FIG . -

Folate Receptor: a Potential Target in Ovarian Cancer
Pteridines 2015; 26(1): 1–12 Review Marie Bartouskova, Bohuslav Melichar and Beatrice Mohelnikova-Duchonova* Folate receptor: a potential target in ovarian cancer Abstract: Ovarian cancer is the most frequent cause of Introduction gynecological cancer-related death. Unfortunately, many patients are diagnosed at an advanced stage and have a Folic acid antagonists have been used in the treatment poor prognosis. The standard treatment for advanced dis- of cancer for more than six decades, since 1948 [1]. Folic ease involves maximal cytoreductive surgery and chemo- acid is a vitamin required for cell metabolism, DNA syn- therapy based on platinum compounds and taxanes. thesis, and repair [2]. Two cellular folate uptake pathways Patients presenting at an advanced stage have a higher have been characterized. The first is mediated by trans- risk of recurrence. The development of drug resistance membrane-reduced folate carriers, which represent the currently represents a major obstacle in the systematic predominant pathway for folate uptake by normal human treatment and, therefore, the discovery of new anticancer cells. Whereas in epithelial cancer cells, membrane pro- agents and approaches should improve the poor progno- teins called folate receptors are often highly expressed and sis of these patients. Folate receptor α is overexpressed in mediate the folate uptake by tumors. Epithelial ovarian epithelial ovarian cancer (EOC), but has limited expres- carcinoma (EOC) has the highest expression of these pro- sion in nonmalignant human tissues. The degree of folate teins, making the folate receptor an attractive candidate receptor expression corresponds with the stage and grade for targeted therapy and targeted drug delivery therapy in of the disease. -
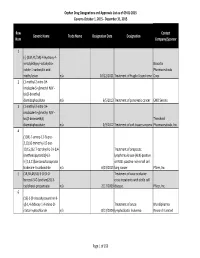
Orphan Drug Designation List
Orphan Drug Designations and Approvals List as of 09‐01‐2015 Governs October 1, 2015 ‐ December 31, 2015 Row Contact Generic Name Trade Name Designation Date Designation Num Company/Sponsor 1 (‐)‐(3aR,4S,7aR)‐4‐Hydroxy‐4‐ m‐tolylethynyl‐octahydro‐ Novartis indole‐1‐carboxylic acid Pharmaceuticals methyl ester n/a 10/12/2011 Treatment of Fragile X syndrome Corp. 2 (1‐methyl‐2‐nitro‐1H‐ imidazole‐5‐yl)methyl N,N'‐ bis(2‐broethyl) diamidophosphate n/a 6/5/2013 Treatment of pancreatic cancer EMD Serono 3 (1‐methyl‐2‐nitro‐1H‐ imidazole‐5‐yl)methyl N,N'‐ bis(2‐bromoethyl) Threshold diamidophosphate n/a 3/9/2012 Treatment of soft tissue sarcoma Pharmaceuticals, Inc. 4 (1OR)‐7‐amino‐12‐fluoro‐ 2,10,16‐trimethyl‐15 oxo‐ 10,15,16,17‐tetrahydro‐2H‐8,4‐ Treatment of anaplastic (metheno)pyrazolo[4,3‐ lymphoma kinase (ALK)‐positive h][2,5,11]benzoxadiazacyclote or ROS1‐positive non‐small cell tradecine‐3‐carbonitrile n/a 6/23/2015 lung cancer Pfizer, Inc. 5 (1R,3R,4R,5S)‐3‐O‐[2‐O‐ Treatment of vaso‐occlusive benzoyl‐3‐O‐(sodium(2S)‐3‐ crisis in patients with sickle cell cyclohexyl‐propanoate‐ n/a 2/17/2009 disease. Pfizer, Inc. 6 (1S)‐1‐(9‐deazahypoxanthin‐9‐ yl)‐1,4‐dideoxy‐1,4‐imino‐D‐ Treatment of acute Mundipharma ribitol‐hydrochloride n/a 8/13/2004 lymphoblastic leukemia Research Limited Page 1 of 359 Orphan Drug Designations and Approvals List as of 09‐01‐2015 Governs October 1, 2015 ‐ December 31, 2015 Row Contact Generic Name Trade Name Designation Date Designation Num Company/Sponsor 7 Treatment of chronic lymphocytic leukemia and related leukemias to include (1S)‐1‐(9‐deazahypoxanthin‐9‐ prolymphocytic leukemia, adult T‐ yl)‐1,4‐dideoxy‐1,4‐imino‐D‐ cell leukemia, and hairy cell Mundipharma ribitol‐hydrochloride n/a 8/10/2004 leukemia Research Ltd. -

WO2020264398A1.Pdf
( (51) International Patent Classification: (71) Applicant: NURIX THERAPEUTICS, INC. [US/US]; A61P35/00 (2006.01) C07D 413/14 (2006.01) 1700 Owens Street, Suite 205, San Francisco, California C07D 401/14 (2006.01) C07D 471/04 (2006.01) 94158 (US). C07D 403/10 (2006.01) A61P 37/00 (2006.01) (72) Inventors: SANDS, Arthur T.; c/o Nurix Therapeutics, C07D 403/12 (2006.01) A61K 31/506 (2006.01) Inc., 1700 Owens Street, Suite 205, San Francisco, Califor¬ C07D 405/14 (2006.01) nia 94158 (US). BENCE, Neil F.; c/o Nurix Therapeutics, (21) International Application Number: Inc., 1700 Owens Street, Suite 205, San Francisco, Califor¬ PCT/US2020/039957 nia 94158 (US). ZAPF, Christoph W.; c/o Nurix Thera¬ peutics, Inc., 1700 Owens Drive, Suite 205, San Francis¬ (22) International Filing Date: co, California 94158 (US). COHEN, Frederick; c/o Nurix 26 June 2020 (26.06.2020) Therapeutics, Inc., 1700 Owens Drive, Suite 205, San Fran¬ (25) Filing Language: English cisco, California 94158 (US). WANG, Chenbo; c/o Nurix Therapeutics, Inc., 1700 Owens Drive, Suite 205, San Fran¬ (26) Publication Language: English cisco, California 94158 (US). CUMMINS, Thomas; c/o (30) Priority Data: Nurix Therapeutics, Inc., 1700 Owens Drive, Suite 205, San 62/866,914 26 June 2019 (26.06.2019) US Francisco, California 94158 (US). TANAKA, Hiroko; c/ 62/880,285 30 July 2019 (30.07.2019) US o Nurix Therapeutics, Inc., 1700 Owens Drive, Suite 205, 62/888,845 19 August 2019 (19.08.2019) US San Francisco, California 94158 (US). SHUNATONA, 62/888,870 19 August 2019 (19.08.2019) US Hunter; c/o Nurix Therapeutics, Inc., 1700 Owens Street, (54) Title: SUBSTITUTED BENZYL-TRIAZOLE COMPOUNDS FOR CBL-B INHIBITION, AND FURTHER USES THEREOF FIG. -

Updates in Cancer for Clinicians
UPDATES IN CANCER FOR CLINICIANS WINTER 2014 Progress in Hematologic Cell Transplantation has been associated with lower myeloid leukemia in first remission.1 rates of relapse in acute myeloid With a median follow up of leukemia and better overall surviving patients exceeding five outcomes. These studies utilized years, the non-relapse mortality fixed oral doses of busulfan, of patients receiving IV busulfan which is associated with up to (IV Bu) was only 12 percent at twentyfold variations in plasma one year and 18 percent at five Edward Copelan, MD, (left) levels. Low plasma levels are years, comparable to reports using Chair of the Department of Hematologic associated with graft rejection and allegedly safer reduced intensity Oncology and Blood Disorders leukemia relapse and high levels regimens, and significantly better with toxicity and transplant-related than the TBI group in the study. Belinda Avalos, MD (right) Vice Chair of the Department of mortality. Improved methods Survival and leukemia free survival Hematologic Oncology and Blood of administration, including the (57 percent at five years for IV Disorders intravenous route and dose Bu) were significantly higher and adjustment of busulfan based on late relapse significantly less Initial studies demonstrating plasma levels, represent important frequent with IV Bu compared a cure of acute leukemia with advances which result in much to TBI. A supportive prospective allogeneic transplantation less variation in plasma levels cohort study in patients with were conducted by Nobel and less toxicity. Drs. Copelan various diagnoses, including Laureate E. Donnall Thomas. and Avalos pioneered the use of AML, and shorter follow up and Total body irradiation (TBI) with busulfan preparative regimens an accompanying commentary cyclophosphamide as preparative and advances in its administration.