Irrigation Water Quality Status in Mulberry Cultivated Areas of Erode
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gobichettipalayam
GOBICHETTIPALAYAM S.No. ROLL No. NAME OF ADVOCATE ADDRESS MEVANI VILLAGE, GOBICHETTIPALAYAM 1 453/1990 ANANDAN M. TALUK, ERODE DIST, PIN - 638313 SELLAKUMARAPALAYAM, 2 528/2011 ANDAVAN S.K. POLAVAKALIPALAYAM PO. GOBI TK. ERODE DT. 638476 27, KANNIKA PARAMESHWARI STREET, 3 1690/1999 ARAVINDAN K. GOBICHETTIPALAYAM, ERODE 70/A, GANDHIPURAM, L. KALLIPATTI, GOBI, 4 3149/2013 ARCCHANA R. ERODE 638452 120,VIVEKANANDAR STREET,SAKTHI NAGAR 5 1185/1997 AYYASAMY K. VIA,P.METTUPALAYAM,BHAVANI TALUK,ERODE-638315 NO.28, BAZAAR ST, GOBICHETTIPALAYAM, 6 1040/1996 BALAMURUGAN G.J. ERODE-638452 NO.29, K.P. EXTENSION, 7 662/1981 BALASUBRAMANIAN G.R. GOBICHETTIPALAYAM. 4/188, M.L.N.HOUSE ARASUR & PO, 8 2068/2000 BALASUBRAMANIAN N. SATHYAMANGALAM TK, ERODE DIST 638454 ANGAYA GOUNDER STREET, KALLIPATTI 9 1412/2013 BARANEEDHARAN K.V. POST, GOBI TALUK, ERODE DIST - 638 505 NO.1, UPPU KIDANGU STREET, 10 879/1995 BASKARAN K.S. GOBICHETTIPALAYAM,ERODE NO.27, CANAL STREET, GOBICHETTIPALAYAM, 11 439/1980 BHARATHI G.K. ERODE - 638452 65/25,PATHIVILAS 12 1877/2000 BIRUNTHA I. STREET,GOBICHETTIPALAYAM,ERODE S.No. ROLL No. NAME OF ADVOCATE ADDRESS 22, AGRAHARA STREET, PERIYA KODIVERI 13 1932/2003 BOOPATHY K.T (PO),SATHYAMANGALAM,ERODE DT-638503. 176, POOSARI VALASU, 14 1893/2001 CHANDRAMOHAN B. PALAVAKALIAPALAYAM POST,GOBICHETTIPALAYAM-638476 DOOR NO:16-B,CKS NAGAR,OPP COURT 15 915/1997 CHANDRASEKARAN K. CAMPUS,GOBICHETTIPALAYAM-638452 NO:20, 4TH STREET MAN NAGAR 16 167/1979 CHINNASWAMY K.S. GOBICHETTIPALAYAM ERODE DIST -638452 D.NO. 254/1, KAMARAJ STREET, D.G.PUDUR 17 1645/2007 CHITHAMBARAM P. PO, NALL ROAD, GOBICHETTI PALAYAM TALUK. -

District Census Handbook, Coimbatore, Part X-A, Series-19
CENSUS OF INDIA, 1971 SER1ES'19 TAMILNADU DISTRICT CENSUS HANDBOOK VILLAGE AND TO"W'N DIRECTORV COIMBATORE K. CHOCKALINGAM "I the Indian· Administrative Service DIRECTOR OF CENSUS OPERATIONS, TAMIL. NADU & PCNDICHERRY 1972 COIMBATORE DISTRICT SCALE Ii 8 4 o Kilometres 8 4 0 8 16 (Area ;: 15, 673 .00 Sq. Km s.) MYSORE SALEM I I / I Chil'lnavadarnpaH'j (5.211) U Vilc;mkuricfti (l,047) fiI Gonopcdni (21,8.f9) IV Sangal'MJr (26»99) V KavundQl'lpglayam (1,062 ) REF ERE NeE VI VlHlrakerQ'om ('.164) VU r.hm."pal'''Y.,m(lJ'.IOll..f'1 District. Headquarters @ VUIK~~""I.y,_(,~,.,.~ MADURAI , IX ....... (5.... 1 I Taluk Headquarters ® X Perur ChMtIpOlo'yotn (5,813) Stale Boundary Xl Kuniarnttthur (25.12!) XU Coimbatore N. M, (:2,,2'6) District Boundary XIII Sing onaflur (1,12.204) XIV Vellolog {I3,.32 } Taluk Boundary XV Kurkhj (41,537) National Highw'!ys_ XVl MadukCff"Oi (I'~' State lfighways '. Roads (B.G.) NAME OF THE AREA IN NO. Of URBAN Railway line (Broad Gauge) TAlUf< SQ. KMS. VlllA,GES CENTRES Railway line (Metre Gauge) (J4..Q.) Bhavan; 1482.54 57 River with Stream GobicheWl><J/oyom 2923.13 151 4 Villages having Population above Weekly ~~~~~th L.C. Number) Erode 1552.55 210 6 M Ohorapufam 221D.'5 86 3 Poot and Telegraph Office I'T Palladarn 1515.77 101 8 Rest House, Travellers Bungalow Ava/lQ!hi 1U7,4I1 /' 73 6 Hospitals H Coimbatone 1368.15 62 20 Urban area PoIl.chi IjJ35.82 117 B Udumolpet 1437.09 93 n' » I\et· No. -

Heavy Metal Content in Irrigation Water Samples of Erode District, Tamil Nadu
Journal of Pharmacognosy and Phytochemistry 2019; 8(5): 1116-1119 E-ISSN: 2278-4136 P-ISSN: 2349-8234 JPP 2019; 8(5): 1116-1119 Heavy metal content in irrigation water samples Received: 16-07-2019 Accepted: 18-08-2019 of Erode district, Tamil Nadu Venu VS Research Scholars, Venu VS, Jothimani P and Prasanthrajan M Department of Sericulture, Forest College and Research Abstract Institute, Mettupalayam, A detailed survey work was carried out in the contamination areas of Erode district, Tamil Nadu, India. A Tamil Nadu Agricultural University, Coimbatore, number of tanneries and textile industries have been established since the past three decades. It is Tamil Nadu, India reported that the effluents from these industries are directly discharged onto the surrounding land, irrigation fields and surface water bodies. As a result, it deteriorates the quality of irrigation water in the Jothimani P study area. The concentrations of various heavy metals namely (Cr, Cd, Ni and Pb) were estimated using Professor, Department of Atomic Absorption Spectrometer. The average concentration of heavy metals in the irrigation water Sericulture, Forest College and ranges from 0.076 - 1.249 ppm, 0.033 - 1.349 ppm, 0.003 - 0.284 ppm and 0.009 - 0.912 ppm for the Research Institute, metals Cr, Cd, Ni and Pb respectively. Mettupalayam, Tamil Nadu Agricultural University, Keywords: Mulberry, irrigation water-quality, heavy metals, tanneries and textile Coimbatore, Tamil Nadu, India Prasanthrajan M Introduction Professor, Department of Mulberry (Morus indica L.) belongs to the family Moraceae a fast growing, deciduous and Sericulture, Forest College and perennial plant. It is the sole food plant of the silkworm (Bombyx mori. -

Tamil Nadu Government Gazette
© [Regd. No. TN/CCN/467/2012-14. GOVERNMENT OF TAMIL NADU [R. Dis. No. 197/2009. 2019 [Price : Rs.17.60 Paise. TAMIL NADU GOVERNMENT GAZETTE PUBLISHED BY AUTHORITY No. 26] CHENNAI, WEDNESDAY, JUNE 26, 2019 Aani 11, Vikari, Thiruvalluvar Aandu – 2050 Part VI—Section 4 Advertisements by private individuals and private institutions CONTENTS PRIVATE ADVERTISEMENTS Pages. Change of Names .. 935-977 Notice .. 977 NOTICE NO LEGAL RESPONSIBILITY IS ACCEPTED FOR THE PUBLICATION OF ADVERTISEMENTS REGARDING CHANGE OF NAME IN THE TAMIL NADU GOVERNMENT GAZETTE. PERSONS NOTIFYING THE CHANGES WILL REMAIN SOLELY RESPONSIBLE FOR THE LEGAL CONSEQUENCES AND ALSO FOR ANY OTHER MISREPRESENTATION, ETC. (By Order) Director of Stationery and Printing. CHANGE OF NAMES 13489. I, V. Muthupriyadharshini, daughter of Thiru M. Arulraj, 13492. My daughter, B. Swathi, born on 28th October 2004 born on 13th October 1998 (native district: Pudukkottai), (native district: Madurai), residing at No. 1/1, Kumaran Street, residing at No. 34/23, Vidathalampatti, Ponnamaravathi, Kamarajapuram, Chinna Anuppanadi, Madurai-625 009, shall R.Palakurichy, Pudukkottai-622 407, shall henceforth be henceforth be known as R. SWATHI known as A. MUTHUPRIYADHARSHINI R. RAJENDRAN V. MUTHUPRIYADHARSHINI Madurai, 17th June 2019. (Father) Pudukkottai, 17th June 2019. 13493. My son, R. Kartick alias Karthik, son of 13490. I, J. Sarmila, wife of Thiru C. Joyalson, born on Thiru S Ramachandran, born on 27th July 2006 (native district: 17th May 1978 (native district: Madurai), residing at No. 56, Madurai), residing at No. 1/121, T. Valayankulam, T. Arasapatti Indirani Nagar, Mudakusalai Main Road, Madurai-625 016, Post, Tirumangalam Taluk, Madurai-625 706, shall henceforth shall henceforth be known as J. -

District Census Handbook, Coimbatore, Part X-B, Vol-I, Series-19
- ©rnlro~l]J~ @Lr LtlroLIDLtl&t) ·1971 SERIES 19 TAMIL NADU PART X-B DISTRICT CENSUS HANDBOOK Village and TOwDwise Pcimary Census Abstract CDIMBATDRE Volume I K. C HOC K A LIN GAM of the Indian ..!!_dmil1istratire Sen-ice DIRECTOR OF CENSUS OPERATIONS, TAMIL NADD AND PONDICHERRY 1972 COIMBATORE DISTRICT SCALE d' S~~1=4~~~o~~~~;;~S~~::::~oii16 Miles Kilometres 8 4 0 8 15 20 (Area: 15,673 . 00 Sq. Kms.) MYSORE It' SALEM NILGIRIS KERALA Index to HUlII bers iu-INSEt ); REFERENCE District Headquarters MADURA I ® IX ~r'Vf" (5,6OS) Taluk Headquarters @ X Perur Ch.tt!palayom (5,813) State Boundary XI Kuniamtttnur (25. 123) xn Coimbatore N. M. (2,256) Distri~t Boundary XIII S;ngonallur (f./2.2(J6J XIV Vellofore (l3,'f32) Taluk Boundary XV Kurkhi (40,537) National Highways XVI Madukarar (1i.I OB1 J State Highways SH Roads NAME OF THE AREA IN NO,OF URBAN Railway line (Broad Gauge) I (~.G.) TALUK SQ, "' ..... S. VILLAGES CENTRES Railway line (Metre Gauge) (M.e.) 8havani 1482.54 57 2 River with Stream Gobichettif'a/ayam 2923.13 151 4 Villages having Population above 5000 (with L.C. Number) Erode (552.55 210 6 Weekly Market. M Oharapurom 2210.95 86 3 Post and Telegraph Office PT Pal/adam 1515.77 101 8 Rest House, Travellers Bungalow R Avan<lsh; 127].411 73 6 Hospitals H Coimbatore Urban area 1368.45 /' 62 20 Pollachi 1835.S;l · 117 8 U<1umalpet f.437.09 93 / .0 CEMiUS- 0' CONTENTS Pages Preface (v)-(vii) figures at a glance (xi)-(xii) District Primary Census Abstract .. -

ANNEXURE 5.8 (CHAPTER V , PARA 25) FORM 9 List of Applications For
ANNEXURE 5.8 (CHAPTER V , PARA 25) FORM 9 List of Applications for inclusion received in Form 6 Designated location identity (where Constituency (Assembly/£Parliamentary): Anthiyur Revision identity applications have been received) 1. List number@ 2. Period of applications (covered in this list) From date To date 16/11/2020 16/11/2020 3. Place of hearing * Serial number$ Date of receipt Name of claimant Name of Place of residence Date of Time of of application Father/Mother/ hearing* hearing* Husband and (Relationship)# 1 16/11/2020 Saihari T A Arulvadivel K V (F) 6/7, kattur street, Thookkanaikanpalayam, , 2 16/11/2020 Gokila vijaychander (H) 167, kemmiyampatti, patlur, , 3 16/11/2020 VIJAYALAKSHMI SURESH (H) 92, ANNANAGAR, KANAKKAMPALAYAM, , £ In case of Union territories having no Legislative Assembly and the State of Jammu and Kashmir Date of exhibition at @ For this revision for this designated location designated location under Date of exhibition at Electoral * Place, time and date of hearings as fixed by electoral registration officer rule 15(b) Registration Officer’s Office under $ Running serial number is to be maintained for each revision for each designated rule 16(b) location # Give relationship as F-Father, M=Mother, and H=Husband within brackets i.e. (F), (M), (H) 23/11/2020 ANNEXURE 5.8 (CHAPTER V , PARA 25) FORM 9 List of Applications for inclusion received in Form 6 Designated location identity (where Constituency (Assembly/£Parliamentary): Anthiyur Revision identity applications have been received) 1. List number@ 2. Period of applications (covered in this list) From date To date 17/11/2020 17/11/2020 3. -

@ S.P.APPARELS LTD. Regd
@ S.P.APPARELS LTD. Regd. omcc: 39 A. Extension Street, Kaikampudur AVINASHI 541 654, Tamil Nadu India. Phnnc #9142957mm 5-mail' spindjaQJapappards cum Web : www.crprcppmrsmm CIN Nu.: Liarorrzzoosmongs 27'h August. 20“) Bombay Stock Exchange Limited National Stock Exchange ot‘lndra Limited Phimze Jeejeebhoy Towers. 'Exchange Plaza', Dalal Street. Bandr-a-Kurla Complex. Bandra (East). Mumbar 7400 001. Mumhar , 400 05l. Scrip Code: 540048 Symbol: SPAL Dear Sirs, Sub: Submission ofAnnual report for the Financial year 2019. Pursuant to Regulation 34“) of SEBl (LODR) Regulations. 2015, we are submitting herewith a copy of Annual Report ofthe Company for the Financial Year ZOl 8—20“) alongwith AGM Notice The said Annual Report ofthe Company for the Financial Year 20] 8-2019 alongwith AGM Notice have been sent to all the members at their email ids registered with the Company / Deposrtory Partrcrpants and dispatched to the members who have not provided their email ids through Registered Post on 27'” August. 20 I9. Further the same has also been uploaded in the Company‘s website at wuwspapparels com. Kindly take the above information on record. Thanking You. For SEApmrels Limited, My\ , KVinodhini Company Secretary and Compliance Officer IA GOVT. OF INDIA RECOGNISED THREE STAR EXPORT HOUSE) S.P. APPARELS LIMITED STITCHING VALUE CREATION ANNUAL REPORT 2018-19 ONE SEAM AT A TIME 21 S.P. APPARELS LIMITED STITCHING VALUE CREATION ANNUAL REPORT 2018-19 ONE SEAM AT A TIME 22 DIRECTORS’ REPORT To The Members, The Directors of your Company are pleased to present the 14th Annual Report on the business and operations of the Company together with the Audited Financial Statements (Standalone & Consolidated) for the year ended 31st March, 2019. -

Draft IEER Scheme 2
CONTENTS Section Description Page No. Executive Summary - 1-2 Section - I : Introduction - 3-5 - Background - 3 - Project Objective - 3 - Project Justification - 3 - Preparation and Objectives of the IEER - 4 - Structure of the IEER - 5 Section - II : Policy, Legal & Regulatory Framework - 6-13 - Environmental (National) - 6 - Social (National) - 9 - Powergrid's Environmental and Social Policy and Procedures - 9 (ESPP) - AIIB’s Environmental and Social Policy and Standards - 10 - Comparison of Powergrid's ESPP and AIIB’s SPF - 10 - International and Regional Agreements and Conventions - 12 Section - III : Project Description - 13-15 - Project Highlights - 13 - Project Scope and Components - 13 - Location of Project Components - 14 - Implementation Plan - 14 Section - IV : Baseline Environment - 16-30 - Physical Resources - 16 - Ecological Environment - 22 - Socio-Economic Environment - 25 Section - V : Analysis of Alternatives and Route/Site Selection - 31-41 - Route Selection - Assessment & Management Process - 31 - Evaluation of Alternatives Routes - 32 - Substations - 41 Section - VI : Potential Impacts, their Evaluation and Management - 42-54 - Impacts and Mitigation due to Project Location - 42 - Environmental and Social Problems due to Design - 50 - Environmental and Social Problems During Construction Phase - 51 - Environmental and Social Problems Resulting from Operation - 53 - Critical Environmental Review Criteria - 54 - Cumulative Impacts - 54 Section - VII : Consultations and Information Disclosure - 55-56 - Consultations - 55 - Information Disclosure - 56 Section - VIII : Implementation Arrangement, Mitigation & Monitoring - 57-73 - Environmental Monitoring Program in Powergrid - 57 - Environmental Management Plan - 58 - EMP Cost Estimate - 73 - Grievance Redressal Mechanism - 73 - Environmental Review - 74 Section - IX : Conclusions - 75 IEER FOR SCHEME 2: AC SYSTEM STRENGTHENING AT PUGALUR END i LIST OF ENCLOSURES S. No. Title/Name Description Annexures 1. -
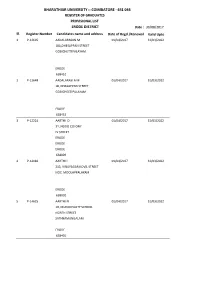
COIMBATORE - 641 046 REGISTER of GRADUATES PROVISIONAL LIST ERODE DISTRICT Date : 30/08/2017 Sl
BHARATHIAR UNIVERSITY :: COIMBATORE - 641 046 REGISTER OF GRADUATES PROVISIONAL LIST ERODE DISTRICT Date : 30/08/2017 Sl. Register Number Candidates name and address Date of Regd./Renewal Valid Upto 1 P-12695 AADALARASAN M 01/04/2017 31/03/2022 18A,DHESAPPAN STREET GOBICHETTIPALAYAM ERODE 638452 2 P-12648 AADALARASI A M 01/04/2017 31/03/2022 18, DHASAPPAN STREET GOBICHEETIPALAYAM ERODE 638452 3 P-12224 AARTHI D 01/04/2017 31/03/2022 31, NGGO COLONY IV STREET ERODE ERODE ERODE 638009 4 P-14082 AARTHI I 01/04/2017 31/03/2022 213, VINAYAGAR KOVIL STREET NO7, MOOLAPPALAYAM ERODE 638002 5 P-14625 AARTHI N 01/04/2017 31/03/2022 10, MUNICIPALITY SCHOOL NORTH STREET SATHYAMANGALAM ERODE 638401 BHARATHIAR UNIVERSITY :: COIMBATORE - 641 046 REGISTER OF GRADUATES PROVISIONAL LIST ERODE DISTRICT Date : 30/08/2017 Sl. Register Number Candidates name and address Date of Regd./Renewal Valid Upto 6 P-14021 AARTHI S 01/04/2017 31/03/2022 1,KAMARAJ VEETHI VINAYAGR KOVIL VEETHI-12 NADARMEEDU ERODE 638002 7 P-13348 ABILA S 01/04/2017 31/03/2022 KITTAMPATTI CHENNAPATTI ANTHIYUR ERODE 638504 8 P-13579 ABINAYA K 01/04/2017 31/03/2022 14 DEVANAMPALAYAM GNANIPALAYAM ERODE 638112 9 P-11828 ABINAYA K 01/04/2017 31/03/2022 SOUTH STREET VATTAKAL VALLASU ERODE ERODE 638012 10 P-11052 ABINAYA K 01/04/2014 31/03/2019 MALAPALAYAM POST PASUR VIA ERODE 638154 BHARATHIAR UNIVERSITY :: COIMBATORE - 641 046 REGISTER OF GRADUATES PROVISIONAL LIST ERODE DISTRICT Date : 30/08/2017 Sl. Register Number Candidates name and address Date of Regd./Renewal Valid Upto 11 P-12919 ABIPRIYANKA -

FORM 11A Designated Location Identity
ANNEXURE 5.11 (CHAPTER V , PARA 25) FORM 11A Designated location identity List of applications for transposition of entry in electoral roll Received in Revision identity (where applications have been Form - 8A received) Constituency (Assembly /£Parliamentary): Anthiyur 1. List number@ 2. Period of receipt of applications (covered in this list) From date To date 09/12/2020 09/12/2020 3. Place of hearing* Serial Date of Details of applicant Details of person whose entry is to be transposed Present place of Date/Time of number of receipt (As given in Part V ordinary residence hearing* application of Form 8A) Name of person Part/Serial EPIC NO. whose entry is to be no. of roll in transposed which name is included 1 09/12/2020 Mahendiran Mahendiran 19 / 107 135/3 ,PAPPATHIKATTU PUDUR ,PUDUR ,KOMARAYANUR , £ In case of Union Territories having no Legislative Assembly and the State of Jammu & Kashmir @ For this revision for this designated location Date of exhibition at designated Date of exhibition at Electoral * Place, time and date of hearing as fixed by electoral registration officer location under rule 15(b) Registration Officer¶s Office under § Running serial number is to be maintained for each revision for each rule 16(b) designated location 22/12/2020 ANNEXURE 5.11 (CHAPTER V , PARA 25) FORM 11A Designated location identity List of applications for transposition of entry in electoral roll Received in Revision identity (where applications have been Form - 8A received) Constituency (Assembly /£Parliamentary): Anthiyur 1. List number@ 2. Period of receipt of applications (covered in this list) From date To date 10/12/2020 10/12/2020 3. -

(400 Kv AC Power Transmission Systems Associated with HVDC Terminal Stations at Pugalur, Tamil Nadu) Main Report
Initial Environmental Examination June 2017 IND: Green Energy Corridor and Grid Strengthening Project (400 kV AC power transmission systems associated with HVDC terminal stations at Pugalur, Tamil Nadu) Main Report Prepared by Power Grid Corporation of India Limited for the Asian Development Bank. This initial environmental examination is a document of the borrower. The views expressed herein do not necessarily represent those of ADB's Board of Directors, Management, or staff, and may be preliminary in nature. Your attention is directed to the “terms of use” section on ADB’s website. In preparing any country program or strategy, financing any project, or by making any designation of or reference to a particular territory or geographic area in this document, the Asian Development Bank does not intend to make any judgments as to the legal or other status of any territory or area. INITIAL ENVIRONMENT EXAMINATION REPORT (IEER) FOR AC SYSTEM STRENGTHENING AT PUGALUR END ENVIRONMENT AND SOCIAL MANAGEMENT POWER GRID CORPORATION OF INDIA LTD. (A GOVERNMENT OF INDIA ENTERPRISE) ESMD/IEER/ AC SYSTEM STRENGTHENING/2017 May’ 2017 CONTENTS Section Description Page No. EXECUTIVE SUMMARY i-ii Section - I : Project Description - 1-3 - Background - 1 - Project Objectives - 2 - Project Justification - 2 - Project Highlights - 2 - Project Scope & Present Study - 3 Section - II : Baseline Data - 4-17 - Tamil Nadu 4 Section- III : Policy, Legal & Regulatory Framework - 18-22 - Environmental - 18 - Social - 21 Section- IV : Approach for Route/Site Selection -
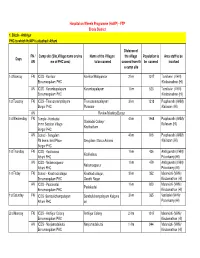
FTP Erode District 1. Block - Anthiyur PHC to Which Howp Is Attached: Athani
Hospital on Wheels Programme (HoWP) - FTP Erode District 1. Block - Anthiyur PHC to which HoWP is attached: Athani Distance of FN / Camp site (Site,Village name and na Name of the Villages the village Population to Area staff to be Days AN me of PHC area) to be covered covered from th be covered involved e camp site 1st Monday FN ICDS - Koviloor Koviloor/Malayanoor 2 km 1017 Tamilselvi (VHN) Ennamangalam PHC /Kirubainathan (HI) AN ICDS - Kurumbapalayam Kurumbapalayam 1 km 523 Tamilselvi (VHN) Ennamangalam PHC /Kirubainathan (HI) 1st Tuesday FN ICDS - Thurusanampalayam Thurusanampalayam/ 3 km 1218 Pusphavathi (ANM) Burgur PHC Puranoor /Kailasam (HI) AN Review Meeting-Burgur 1st Wednesday FN Temple - Konkadai 4 km 1968 Pusphavathi (ANM)/ Gonkadai Colony/ Immn Session Village Kailasam (HI) Kovilnatham Burgur PHC AN School - Sengulam 4 km 816 Pusphavathi (ANM) FN Immn. held Place Sengulam /Oosur Aalanai /Kailasam (HI) Burgur PHC 1st Thursday FN ICDS - Koolivalasu 1 km 436 Ambigavathi (VHN)/ Koolivalasu Athani PHC Palanisamy (HI) AN ICDS - Nallamoopanur 1 km 479 Ambigavathi (VHN)/ Nallamoopanur Athani PHC Palanisamy (HI) 1st Friday FN School - Kinathadisolagur Kinathadisolagur, 3 km 352 Malarvizhi (VHN) / Ennamangalam PHC Gandhi Nagar Kirubainathan (HI) AN ICDS - Paalakudai 1 km 820 Malarvizhi (VHN) / Paalakudai Ennamangalam PHC Kirubainathan (HI) 1st Saturday FN ICDS -Sembulichampalayam Sembulichampalayam Kalguva 3 km 365 Vetriselvi (VHN)/ Athani PHC ari Palanisamy (HI) 2nd Monday FN ICDS - Anthiyur Colony Anthiyur Colony 2 Km 1017 Malarvizhi