Modeling Prehistoric Health in the Middle Cumberland Region of Tennessee: Mississippian Populations on the Threshold of Collapse
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mortuary Patterns in West-Central Tennessee: Contextualizing Historic Field Data from Nine Mississippian Period Sites
Illinois State University ISU ReD: Research and eData Theses and Dissertations 4-6-2018 Mortuary Patterns In West-Central Tennessee: Contextualizing Historic Field Data From Nine Mississippian Period Sites Brooke Adele Wamsley Illinois State University, [email protected] Follow this and additional works at: https://ir.library.illinoisstate.edu/etd Part of the Biological and Physical Anthropology Commons, and the History of Art, Architecture, and Archaeology Commons Recommended Citation Wamsley, Brooke Adele, "Mortuary Patterns In West-Central Tennessee: Contextualizing Historic Field Data From Nine Mississippian Period Sites" (2018). Theses and Dissertations. 896. https://ir.library.illinoisstate.edu/etd/896 This Thesis is brought to you for free and open access by ISU ReD: Research and eData. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of ISU ReD: Research and eData. For more information, please contact [email protected]. MORTUARY PATTERNS IN WEST-CENTRAL TENNESSEE: CONTEXTUALIZING HISTORIC FIELD DATA FROM NINE MISSISSIPPIAN PERIOD SITES Brooke A. Wamsley 156 pages Middle Mississippian is a both a cultural and temporal (1200 CE – 1400 CE) archaeological context of Midwestern North America. This cultural tradition is associated with mound building, specific art motifs, arguably stratified societies, intensive agriculture, and specific ritual/mortuary practices. Burial sites can be very valuable to archaeologists because of the purposeful interaction between the living and the deceased and reconstruction of cultural elements such as social identity and group membership. While American archaeology continues to be fieldwork-focused, there are a considerable amount of cultural resources housed in museum collections that could provide data for research into pre-Columbian life-ways in North America. -

SEAC Bulletin 58.Pdf
SOUTHEASTERN ARCHAEOLOGICAL CONFERENCE PROCEEDINGS OF THE 72ND ANNUAL MEETING NOVEMBER 18-21, 2015 NASHVILLE, TENNESSEE BULLETIN 58 SOUTHEASTERN ARCHAEOLOGICAL CONFERENCE BULLETIN 58 PROCEEDINGS OF THE 72ND ANNUAL MEETING NOVEMBER 18-21, 2015 DOUBLETREE BY HILTON DOWNTOWN NASHVILLE, TENNESSEE Organized by: Kevin E. Smith, Aaron Deter-Wolf, Phillip Hodge, Shannon Hodge, Sarah Levithol, Michael C. Moore, and Tanya M. Peres Hosted by: Department of Sociology and Anthropology, Middle Tennessee State University Division of Archaeology, Tennessee Department of Environment and Conservation Office of Social and Cultural Resources, Tennessee Department of Transportation iii Cover: Sellars Mississippian Ancestral Pair. Left: McClung Museum of Natural History and Culture; Right: John C. Waggoner, Jr. Photographs by David H. Dye Printing of the Southeastern Archaeological Conference Bulletin 58 – 2015 Funded by Tennessee Department of Environment and Conservation, Authorization No. 327420, 750 copies. This public document was promulgated at a cost of $4.08 per copy. October 2015. Pursuant to the State of Tennessee’s Policy of non-discrimination, the Tennessee Department of Environment and Conservation does not discriminate on the basis of race, sex, religion, color, national or ethnic origin, age, disability, or military service in its policies, or in the admission or access to, or treatment or employment in its programs, services or activities. Equal Employment Opportunity/Affirmative Action inquiries or complaints should be directed to the Tennessee Department of Environment and Conservation, EEO/AA Coordinator, Office of General Counsel, 312 Rosa L. Parks Avenue, 2nd floor, William R. Snodgrass Tennessee Tower, Nashville, TN 37243, 1-888-867-7455. ADA inquiries or complaints should be directed to the ADA Coordinator, Human Resources Division, 312 Rosa L. -

Volume 16 Number 1 Fall 2012
Fall 2012 KENTUCKYARCHAEOLOGY I The Newsletter of the Kentucky Organization of Professional Archaeologists TABLEOF CONTENTS in a timely manner is a key issue for members as well as those who submit items. We obtained only Editor's Note .... 1 one submittal that was used to generate this publication. This diminutive submittal rate may Presidential Corner .... 2 reflect the snail like speed currently exhibited with getting this publication that results in members to Feature Topic: A Commentary ... .4 reject submitting their work. Perhaps it may also be from the overall need for members to become more Paper and Poster Abstracts Presented at the 29th involved, more out spoken on their work, and ideas Annual Kentucky Heritage Council (KHC) about Kentucky archaeology. Kit Wesler in his Conference .... 8 President's Corner offers several ideas on how members can help address this need. We challenge members to send in ideas on what is important to News & Announcements .... 14 them about Kentucky archaeology as well as short Native American Day .... 14 articles, interesting artifact descriptions, or Papers, Posters, and Research .... 15 explanations regarding how they contribute to KHC Abstracts .... 15 archaeology within the state. We also challenge Manuscripts ... 15 members employed by cultural resource Memorial Service ... 15 management companies and institutes working in Kentucky to submit recent abstracts of data recovery KyOPA Membership Summary .... 15 projects or summaries of significant finds relevant to the state for publication in the KyOPA Newsletter. Area of Interest. ... 16 In the not too distant past, the KyOPA Newsletter published data recovery abstracts and we seek to KyOPA Officers and Board Members ... -

APR 2 7 200I National Register of Historic Places Multiple Property Documentation Form
NPS Form 10^900-b OMB No. 1024-<X (Revised March 1992) United Skates Department of the Interior iatlonal Park Service APR 2 7 200I National Register of Historic Places Multiple Property Documentation Form This form Is used for documenting multiple property groups relating to one or several historic contexts. See Instructions in How to Complete t MuMple Property Documentation Form (National Register Bulletin 168). Complete each Item by entering the requested information. For additional space, use continuation sheets (Form 10-900-a). Use a typewriter, word processor, or computer to complete all Items. _X_ New Submission __ Amended Submission A. Name of Multiple Property Listing Native American Rock Art Sites of Illinois B. Associated Historic Contexts_______________________________ (Name each associated historic context, identifying theme, geographical area, and chronological period for each.) Native American Rock Art of Illinois (7000 B.C. - ca. A. D. 1835) C. Form Prepared by name/title Mark J. Wagner, Staff Archaeologist organization Center for Archaeological Investigations dat0 5/15/2000 Southern Illinois University street & number Mailcode 4527 telephone (618) 453-5035 city or town Carbondale state IL zip code 62901-4527 D. Certification As the designated authority under the National Historic Preservation Act of 1966, as amended, I hereby certify that this documentation form meets the National Register documentation standards and sets forth requirements for the listing of related properties consistent with the National Register criteria. This submission meets the procedural and professional requirements set forth In 36 CFR Part 60 and the Secretary of the Interior's Standards and Guidelines for Archeology and Historic Preservation. -

Tennessee Archaeology Is Published Semi-Annually in Electronic Print Format by the Tennessee Council for Professional Archaeology
TTEENNNNEESSSSEEEE AARRCCHHAAEEOOLLOOGGYY Volume 3 Spring 2008 Number 1 EDITORIAL COORDINATORS Michael C. Moore TTEENNNNEESSSSEEEE AARRCCHHAAEEOOLLOOGGYY Tennessee Division of Archaeology Kevin E. Smith Middle Tennessee State University VOLUME 3 Spring 2008 NUMBER 1 EDITORIAL ADVISORY COMMITTEE David Anderson 1 EDITORS CORNER University of Tennessee ARTICLES Patrick Cummins Alliance for Native American Indian Rights 3 Evidence for Early Mississippian Settlement Aaron Deter-Wolf of the Nashville Basin: Archaeological Division of Archaeology Explorations at the Spencer Site (40DV191) W. STEVEN SPEARS, MICHAEL C. MOORE, AND Jay Franklin KEVIN E. SMITH East Tennessee State University RESEARCH REPORTS Phillip Hodge Department of Transportation 25 A Surface Collection from the Kirk Point Site Zada Law (40HS174), Humphreys County, Tennessee Ashland City, Tennessee CHARLES H. MCNUTT, JOHN B. BROSTER, AND MARK R. NORTON Larry McKee TRC, Inc. 77 Two Mississippian Burial Clusters at Katherine Mickelson Travellers’ Rest, Davidson County, Rhodes College Tennessee DANIEL SUMNER ALLEN IV Sarah Sherwood University of Tennessee 87 Luminescence Dates and Woodland Ceramics from Rock Shelters on the Upper Lynne Sullivan Frank H. McClung Museum Cumberland Plateau of Tennessee JAY D. FRANKLIN Guy Weaver Weaver and Associates LLC Tennessee Archaeology is published semi-annually in electronic print format by the Tennessee Council for Professional Archaeology. Correspondence about manuscripts for the journal should be addressed to Michael C. Moore, Tennessee Division of Archaeology, Cole Building #3, 1216 Foster Avenue, Nashville TN 37243. The Tennessee Council for Professional Archaeology disclaims responsibility for statements, whether fact or of opinion, made by contributors. On the Cover: Human effigy bowl from Travellers’ Rest, Courtesy, Aaron Deter-Wolf EDITORS CORNER Welcome to the fifth issue of Tennessee Archaeology. -

2016 Athens, Georgia
SOUTHEASTERN ARCHAEOLOGICAL CONFERENCE PROCEEDINGS & ABSTRACTS OF THE 73RD ANNUAL MEETING OCTOBER 26-29, 2016 ATHENS, GEORGIA BULLETIN 59 2016 BULLETIN 59 2016 PROCEEDINGS & ABSTRACTS OF THE 73RD ANNUAL MEETING OCTOBER 26-29, 2016 THE CLASSIC CENTER ATHENS, GEORGIA Meeting Organizer: Edited by: Hosted by: Cover: © Southeastern Archaeological Conference 2016 TABLE OF CONTENTS THE CLASSIC CENTER FLOOR PLAN……………………………………………………...……………………..…... PREFACE AND ACKNOWLEDGEMENTS…………………………………………………………………….…..……. LIST OF DONORS……………………………………………………………………………………………….…..……. SPECIAL THANKS………………………………………………………………………………………….….....……….. SEAC AT A GLANCE……………………………………………………………………………………….……….....…. GENERAL INFORMATION & SPECIAL EVENTS SCHEDULE…………………….……………………..…………... PROGRAM WEDNESDAY, OCTOBER 26…………………………………………………………………………..……. THURSDAY, OCTOBER 27……………………………………………………………………………...…...13 FRIDAY, OCTOBER 28TH……………………………………………………………….……………....…..21 SATURDAY, OCTOBER 29TH…………………………………………………………….…………....…...28 STUDENT PAPER COMPETITION ENTRIES…………………………………………………………………..………. ABSTRACTS OF SYMPOSIA AND PANELS……………………………………………………………..…………….. ABSTRACTS OF WORKSHOPS…………………………………………………………………………...…………….. ABSTRACTS OF SEAC STUDENT AFFAIRS LUNCHEON……………………………………………..…..……….. SEAC LIFETIME ACHIEVEMENT AWARDS FOR 2016…………………….……………….…….…………………. Southeastern Archaeological Conference Bulletin 59, 2016 ConferenceRooms CLASSIC CENTERFLOOR PLAN 6 73rd Annual Meeting, Athens, Georgia EVENT LOCATIONS Baldwin Hall Baldwin Hall 7 Southeastern Archaeological Conference Bulletin -

Faunal Remains from an Archaic Period Cave in the Southeastern United States
Journal of Archaeological Science: Reports 8 (2016) 187–199 Contents lists available at ScienceDirect Journal of Archaeological Science: Reports journal homepage: www.elsevier.com/locate/jasrep Faunal remains from an archaic period cave in the Southeastern United States Tanya M. Peres a, Aaron Deter-Wolf b, Joey Keasler c, Shannon Chappell Hodge c a Department of Anthropology, Florida State University, 1847 W. Tennessee Street, Tallahassee, FL 32306, USA b Tennessee Division of Archaeology, Cole Building #3, 1216 Foster Ave., Nashville, TN 37243, USA c Department of Sociology and Anthropology, Box 10, 1301 E. Main Street, Middle Tennessee State University, Murfreesboro, TN, 37132, USA article info abstract Article history: Ancient Native American use of caves in the Eastern Woodlands occurred throughout the entire span of regional Received 18 December 2015 prehistory; however, the ways that these natural features were used varied considerably over time. To date only Received in revised form 31 May 2016 25 cave sites containing deposits dated to the Archaic period (ca. 10,000–3000 B.P.) are recorded in the state of Accepted 3 June 2016 Tennessee, representing just 0.4% of the total known Archaic sites. In 2014 the authors conducted a salvage op- eration, bucket auger survey, and limited testing at the site of Black Cat Cave (40RD299) in Rutherford County, Tennessee to assess looting damage and assist in the installation of a security gate across the cave entrance. Keywords: fi fi Cave These investigations identi ed Black Cat Cave as the site of signi cant mortuary activity during the Middle Archa- Archaic period ic (ca. -
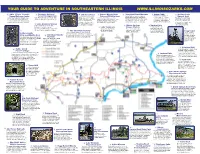
Hardin County Area Map.Pdf
YOUR GUIDE TO ADVENTURE IN SOUTHEASTERN ILLINOIS WWW.ILLINOISOZARKS.COM 1 Ohio River Scenic 4 Shawnee National 3 Old Stone Face 6 Sahara Woods State 7 Stonefort Depot Museum 11 Camp Cadiz 15 Golden Circle This former coal mining area Byway Welcome Center Forest Headquarters A ½ mile moderately strenuous Fish and Wildlife Area Built in 1890, this former railroad depot Natural Arch is now a 2,300 acre state park On the corner in downtown Equality. View Main office for the national forest with visitor trail takes you to scenic vistas This former coal mining area is now a is a step back in time with old signs from This unique rock arch forms a managed for hunting and fishing. their extensive collection of artifacts from information, displays and souvenirs for sale. and one of the finest and natural 2,300 acre state park managed for hunting railroad companies and former businesses, natural amphitheater that was Plans are being developed for the salt well industry while taking advantage stone face rock formations. and fishing. Plans are being developed tools and machines from the heyday of the secret meeting place of a off-road vehicle recreation trails. of indoor restrooms and visitor’s information. Continue on the Crest Trail to for off-road vehicle recreation trails. railroads and telegraphs are on display. group of southern sympathizers, the Tecumseh Statue at Glen the Knights of the Golden 42 Lake Glendale Stables O Jones Lake 3 miles away. Circle, during the Civil War. Saddle up and enjoy an unforgettable 40 Hidden Springs 33 Burden Falls horseback ride no matter what your 20 Lake Tecumseh Ranger Station During wet weather, an intermittent stream spills experience level. -

4.0 a Guide to Warrior Suits 4.1 the Basic Feather Costume
4.0 A GUIDE TO WARRIOR SUITS Here follows a broad outline of the various warrior suits that were known to be associated with the Aztecs. It should be noted that all of these showy feather suits were available to the noblemen only, and could never be worn by the common man. The prime source of information for the following chapter is from the tribute lists in the Codex Mendoza and the Matricula de Tributos, the suit and banner lists in the Primeros Memoriales plus some commentary in Duran's Book of the Gods, History of the Indies and some of the Florentine Codex. The first two give clear examples of many different types of war suits, as well as a defined list of warrior and priest suits with their associated rank. Unfortunately they do not show all the suit types possible, nor do they explain what several of the suit types sent in tribute were for. Trying to mesh these sources is not neat, and some interpretation is required. While Chapter 3 examined the tribute from various provinces on a province by province basis, this chapter concentrates only on the warrior suit types as individual topics. The Mendoza Noble Warrior List (Section 4.2) and Priest Warrior List (Section 4.3) are further expanded upon with information from the tribute lists and then any other primary sources with relevant information or images. These lists are then followed up by a list of suit types not covered by either of these two lists (Section 4.4.) This section of the documentation does not cover the organisational or ranking levels of the suits except as a method for listing the suits for discussion. -

The Late Mississippian Period (AD 1350-1500) - Draft
SECTION IV: The Mississippian Period in Tennessee Chapter 12: The Late Mississippian Period (AD 1350-1500) - Draft By Michaelyn Harle, Shannon D. Koerner, and Bobby R. Braly 1 Introduction Throughout the Mississippian world this time period appears to be a time of great social change. In eastern Tennessee, Dallas Phase sites further elaborated on the Mississippian lifeway, becoming highly organized and home to political leaders. Settlements were sometimes quite extensive (i.e., the Dallas, Toqua, and Ledford Island sites with deep middens, often a palisade wall, sometimes with bastions, densely packed domestic structures, and human interments throughout the village area and also in mounds. Elsewhere in the region, there is evidence that much of West Tennessee and parts of the Cumberland-Tennessee valley were either abandoned by Mississippian societies or so fundamentally reorganized that they were rendered archaeologically invisible. This abandonment appears to be part of a larger regional trend of large portions of the Central Mississippi Valley, often referred to as the vacant quarter. A number of motives for this abandonment have been provided including the dissolution of Cahokia, increased intra- regional warfare, and environmental shifts associated with the onslaught of the Little Ice Age (Meeks 2006; Cobb and Butler 2002; Williams 1983, 1990). This two sides of the continium is important since it gives us a more microscopic glimpse of what was being played out in the larger pan-Mississippian stage. Regional and temporal refinements that are currently in progress gives us a unique perspective into the similarities and differences in which Tennessee Mississippian societies reacted to this unstable period. -

One Hundred Years of Investigations at the Linn Site in Southern Illinois
University of South Carolina Scholar Commons Faculty Publications Anthropology, Department of 1991 One Hundred Years of Investigations at the Linn Site in Southern Illinois Charles R. Cobb University of South Carolina - Columbia, [email protected] Follow this and additional works at: https://scholarcommons.sc.edu/anth_facpub Part of the Anthropology Commons Publication Info Published in Illinois Archaeology, Volume 3, Issue 1, 1991, pages 56-76. © Illinois Archaeology 1991, The Illinois State Archaeological Survey. This Article is brought to you by the Anthropology, Department of at Scholar Commons. It has been accepted for inclusion in Faculty Publications by an authorized administrator of Scholar Commons. For more information, please contact [email protected]. One Hundred Years of Investigations at the Linn Site in Southern Illinois Charles R. Cobb The Linn site represents one of the major Mississippian occupations in the Mississippi River floodplain of southwestern Illinois. The multiple mound center has received sporadic professional attention over the years dating from Bureau of Ethnology inves tigations in the latter part of the nineteenth century; however, little work by modern standards has been conducted at the site. Consequently, very little is known about the Linn site and its relationship to other Mississippian traditions in surrounding regions. This study synthesizes dtlta from past research on the site, the results of which indicate that the Linn site likely played a major role in late prehistoric developments in the central Mississippi River valley. The region of southwestern illinois that extends from the southern end of the American Bottom to the confluence of the Mississippi and Ohio rivers is known to be home to a number of important Mississippian sites, including mound centers, special-use sites (e.g., chert quarries and workshops), as well as the usual array of hamlets, farmsteads, and villages. -

Discover Illinois Archaeology
Discover Illinois Archaeology ILLINOIS ASSOCIATION FOR ADVANCEMENT OF ARCHAEOLOGY ILLINOIS ARCHAEOLOGICAL SURVEY Discover Illinois Archaeology Illinois’ rich cultural heritage began more collaborative effort by 18 archaeologists from than 12,000 years ago with the arrival of the across the state, with a major contribution by ancestors of today’s Native Americans. We learn Design Editor Kelvin Sampson. Along with sum- about them through investigations of the remains maries of each cultural period and highlights of they left behind, which range from monumental regional archaeological research, we include a earthworks with large river-valley settlements to short list of internet and print resources. A more a fragment of an ancient stone tool. After the extensive reading list can be found at the Illinois arrival of European explorers in the late 1600s, a Association for Advancement of Archaeology succession of diverse settlers added to our cul- web site www.museum.state.il.us/iaaa/DIA.pdf. tural heritage, leading to our modern urban com- We hope that by reading this summary of munities and the landscape we see today. Ar- Illinois archaeology, visiting a nearby archaeo- chaeological studies allow us to reconstruct past logical site or museum exhibit, and participating environments and ways of life, study the rela- in Illinois Archaeology Awareness Month pro- tionship between people of various cultures, and grams each September, you will become actively investigate how and why cultures rise and fall. engaged in Illinois’ diverse past and DISCOVER DISCOVER ILLINOIS ARCHAEOLOGY, ILLINOIS ARCHAEOLOGY. summarizing Illinois culture history, is truly a Alice Berkson Michael D. Wiant IIILLINOIS AAASSOCIATION FOR CONTENTS AAADVANCEMENT OF INTRODUCTION.