Characterization of the DNA Binding Properties of CST (CTC1-STN1-TEN1) and Their
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Architecture of a Eukaryotic Replisome
The Architecture of a Eukaryotic Replisome Jingchuan Sun1,2, Yi Shi3, Roxana E. Georgescu3,4, Zuanning Yuan1,2, Brian T. Chait3, Huilin Li*1,2, Michael E. O’Donnell*3,4 1 Biosciences Department, Brookhaven National Laboratory, Upton, New York, USA 2 Department of Biochemistry & Cell Biology, Stony Brook University, Stony Brook, New York, USA. 3 The Rockefeller University, 1230 York Avenue, New York, New York, USA. 4 Howard Hughes Medical Institute *Correspondence and requests for materials should be addressed to M.O.D. ([email protected]) or H.L. ([email protected]) ABSTRACT At the eukaryotic DNA replication fork, it is widely believed that the Cdc45-Mcm2-7-GINS (CMG) helicase leads the way in front to unwind DNA, and that DNA polymerases (Pol) trail behind the helicase. Here we use single particle electron microscopy to directly image a replisome. Contrary to expectations, the leading strand Pol ε is positioned ahead of CMG helicase, while Ctf4 and the lagging strand Pol α-primase (Pol α) are behind the helicase. This unexpected architecture indicates that the leading strand DNA travels a long distance before reaching Pol ε, it first threads through the Mcm2-7 ring, then makes a U-turn at the bottom to reach Pol ε at the top of CMG. Our work reveals an unexpected configuration of the eukaryotic replisome, suggests possible reasons for this architecture, and provides a basis for further structural and biochemical replisome studies. INTRODUCTION DNA is replicated by a multi-protein machinery referred to as a replisome 1,2. Replisomes contain a helicase to unwind DNA, DNA polymerases that synthesize the leading and lagging strands, and a primase that makes short primed sites to initiate DNA synthesis on both strands. -

Orpl, a Member of the Cdcl8/Cdc6 Family of S-Phase Regulators, Is Homologous to a Component of the Origin Recognition Complex M
Proc. Natl. Acad. Sci. USA Vol. 92, pp. 12475-12479, December 1995 Genetics Orpl, a member of the Cdcl8/Cdc6 family of S-phase regulators, is homologous to a component of the origin recognition complex M. MuzI-FALCONI AND THOMAS J. KELLY Department of Molecular Biology and Genetics, Johns Hopkins University School of Medicine, Baltimore, MD 21205 Contributed by Thomas J. Kelly, September 11, 1995 ABSTRACT cdc18+ of Schizosaccharomyces pombe is a is the cdcJ8+ gene (1, 15). Expression of cdcJ8+ from a periodically expressed gene that is required for entry into S heterologous promoter is sufficient to rescue the lethality of a phase and for the coordination of S phase with mitosis. cdc18+ conditional temperature-sensitive (ts) cdc O's mutant. The is related to the Saccharomyces cerevisiae gene CDC6, which has cdcJ8+ gene product, a 65-kDa protein, is essential for the also been implicated in the control of DNA replication. We GI/S transition. Moreover, p65cdclS is a highly labile protein have identified a new Sch. pombe gene, orpl1, that encodes an whose expression is confined to a narrow window at the G,/S 80-kDa protein with amino acid sequence motifs conserved in boundary (unpublished data). These properties are consistent the Cdc18 and Cdc6 proteins. Genetic analysis indicates that with the hypothesis that Cdc18 may play an important role in orpi + is essential for viability. Germinating spores lacking the regulating the initiation of DNA replication at S phase. The orpl + gene are capable of undergoing one or more rounds of Cdc18 protein is homologous to the budding yeast Cdc6 DNA replication but fail to progress further, arresting as long protein, which may have a similar function (16). -

Derepression of Htert Gene Expression Promotes Escape from Oncogene-Induced Cellular Senescence
Derepression of hTERT gene expression promotes escape from oncogene-induced cellular senescence Priyanka L. Patela, Anitha Surama, Neena Miranib, Oliver Bischofc,d, and Utz Herbiga,e,1 aNew Jersey Medical School-Cancer Center, Rutgers Biomedical and Health Sciences, Newark, NJ 07103; bDepartment of Pathology and Laboratory Medicine, New Jersey Medical School, Rutgers Biomedical and Health Sciences, Newark, NJ 07103; cNuclear Organization and Oncogenesis Unit, Department of Cell Biology and Infection, Institut Pasteur, 75015 Paris, France; dINSERM U993, F-75015 Paris, France; and eDepartment of Microbiology, Biochemistry, and Molecular Genetics, Rutgers Biomedical and Health Sciences, Rutgers University, Newark, NJ 07103 Edited by Victoria Lundblad, Salk Institute for Biological Studies, La Jolla, CA, and approved June 27, 2016 (received for review February 11, 2016) Oncogene-induced senescence (OIS) is a critical tumor-suppressing that occur primarily at fragile sites. The ensuing DNA damage mechanism that restrains cancer progression at premalignant stages, response (DDR) triggers OIS, thereby arresting cells within a few in part by causing telomere dysfunction. Currently it is unknown cell-division cycles after oncogene expression (8, 9). Although most whether this proliferative arrest presents a stable and therefore DSBs in arrested cells are eventually resolved by cellular DSB irreversible barrier to cancer progression. Here we demonstrate that repair processes, some persist and consequently convert the other- cells frequently escape OIS induced by oncogenic H-Ras and B-Raf, wise transient DDR into a more permanent growth arrest. We and after a prolonged period in the senescence arrested state. Cells that others have demonstrated that the persistent DDR is primarily had escaped senescence displayed high oncogene expression levels, telomeric, triggered by irreparable telomeric DSBs (1, 10, 11). -

Association of ORC with Replication Origins 2013
Journal of Cell Science 112, 2011-2018 (1999) 2011 Printed in Great Britain © The Company of Biologists Limited 1999 JCS0252 Changes in association of the Xenopus origin recognition complex with chromatin on licensing of replication origins Alison Rowles1,*, Shusuke Tada1,2,‡ and J. Julian Blow1,2,‡,§ 1ICRF Clare Hall Laboratories, South Mimms, Potters Bar, Herts EN6 3LD, UK 2CRC Chromosome Replication Research Group, Department of Biochemistry, University of Dundee, Dundee DD1 5EH, Scotland, UK *Present address: Department of Neuroscience, SmithKline Beecham Pharmaceuticals, New Frontiers Science Park North, Harlow, Essex CM19 5AW, UK ‡Present address: CRC Chromosome Replication Research Group, Department of Biochemistry, University of Dundee, Dundee DD1 5EH, Scotland, UK §Author for correspondence Accepted 29 March; published on WWW 26 May 1999 SUMMARY During late mitosis and early G1, a series of proteins are chromatin, as evidenced by its resistance to elution by 200 assembled onto replication origins that results in them mM salt, and this state persisted when XCdc6 was assembled becoming ‘licensed’ for replication in the subsequent S phase. onto the chromatin. As a consequence of origins becoming In Xenopus this first involves the assembly onto chromatin of licensed the association of XOrc1 and XCdc6 with chromatin the Xenopus origin recognition complex XORC, and then was destabilised, and XOrc1 became susceptible to removal XCdc6, and finally the RLF-M component of the replication from chromatin by exposure to either high salt or high Cdk licensing system. In this paper we examine changes in the way levels. At this stage the essential function for XORC and that XORC associates with chromatin in the Xenopus cell- XCdc6 in DNA replication had already been fulfilled. -

The AAA+ Proteins Pontin and Reptin Enter Adult Age: from Understanding Their Basic Biology to the Identification of Selective Inhibitors
PERSPECTIVE published: 05 May 2015 doi: 10.3389/fmolb.2015.00017 The AAA+ proteins Pontin and Reptin enter adult age: from understanding their basic biology to the identification of selective inhibitors Pedro M. Matias 1, 2*, Sung Hee Baek 3, Tiago M. Bandeiras 2, Anindya Dutta 4, Walid A. Houry 5, Oscar Llorca 6 and Jean Rosenbaum 7, 8* 1 Instituto de Tecnologia Química e Biológica António Xavier, Universidade Nova de Lisboa, Oeiras, Portugal, 2 Instituto de 3 Edited by: Biologia Experimental e Tecnológica, Oeiras, Portugal, Creative Research Initiative Center for Chromatin Dynamics, School 4 Rui Joaquim Sousa, of Biological Sciences, Seoul National University, Seoul, South Korea, Department of Biochemistry and Molecular Genetics, 5 The University of Texas Health University of Virginia, Charlottesville, VA, USA, Department of Biochemistry, University of Toronto, Toronto, ON, Canada, 6 Science Center, USA Centro de Investigaciones Biológicas, Consejo Superior de Investigaciones Científicas (Spanish National Research Council, CSIC), Madrid, Spain, 7 INSERM, U1053, Bordeaux, France, 8 Groupe de Recherches pour l’Etude du Foie, Université de Reviewed by: Bordeaux, Bordeaux, France Eileen M. Lafer, University of Texas Health Science Center at San Antonio, USA Pontin and Reptin are related partner proteins belonging to the AAA+ (ATPases Pierre Goloubinoff, Associated with various cellular Activities) family. They are implicated in multiple and University of Lausanne, Switzerland seemingly unrelated processes encompassing the regulation of gene transcription, the *Correspondence: Pedro M. Matias, remodeling of chromatin, DNA damage sensing and repair, and the assembly of protein Instituto de Tecnologia Química e and ribonucleoprotein complexes, among others. The 2nd International Workshop Biológica António Xavier, Universidade Nova de Lisboa, Av. -

Sumoylation of Pontin Chromatin-Remodeling Complex Reveals a Signal Integration Code in Prostate Cancer Cells
SUMOylation of pontin chromatin-remodeling complex reveals a signal integration code in prostate cancer cells Jung Hwa Kim*†, Ji Min Lee*, Hye Jin Nam*, Hee June Choi*, Jung Woo Yang*, Jason S. Lee*, Mi Hyang Kim‡, Su-Il Kim‡, Chin Ha Chung*, Keun Il Kim§, and Sung Hee Baek*¶ *Department of Biological Sciences, Research Center for Functional Cellulomics and ‡School of Agricultural Biotechnology, Seoul National University, Seoul 151-742, South Korea; §Department of Biological Sciences, Research Center for Women’s Disease, Sookmyung Women’s University, Seoul 140-742, South Korea; and †Department of Medical Sciences, Inha University, Incheon 402-751, South Korea Communicated by Michael G. Rosenfeld, University of California at San Diego, La Jolla, CA, November 6, 2007 (received for review July 20, 2007) Posttranslational modification by small ubiquitin-like modifier mammals, they constitute parts of the Tip60 coactivator complex, (SUMO) controls diverse cellular functions of transcription factors which has intrinsic histone acetyltransferase activity (8). In ze- and coregulators and participates in various cellular processes brafish embryos, the reptin/pontin ratio serves to regulate heart including signal transduction and transcriptional regulation. Here, growth during development via the -catenin pathway (9). we report that pontin, a component of chromatin-remodeling Posttranslational modification of proteins plays an important role complexes, is SUMO-modified, and that SUMOylation of pontin is in the functional regulation of transcriptional coregulators. Numer- an active control mechanism for the transcriptional regulation of ous enzymatic activities have been demonstrated to be associated pontin on androgen-receptor target genes in prostate cancer cells. with coregulator complexes, including histone acetylation/ Biochemical purification of pontin-containing complexes revealed deacetylation, phosphorylation/dephosphorylation, ubiquitination, the presence of the Ubc9 SUMO-conjugating enzyme that underlies and SUMOylation (10). -

In Vivo Interactions of Archaeal Cdc6 Orc1 and Minichromosome
In vivo interactions of archaeal Cdc6͞Orc1 and minichromosome maintenance proteins with the replication origin Fujihiko Matsunaga*, Patrick Forterre*, Yoshizumi Ishino†, and Hannu Myllykallio*§ *Institut de Ge´ne´ tique et Microbiologie, Universite´de Paris-Sud, 91405 Orsay, France; and †Department of Molecular Biology, Biomolecular Engineering Research Institute, 6-2-3 Furuedai, Suita, Osaka 565-0874, Japan Communicated by Bruce W. Stillman, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, July 25, 2001 (received for review May 25, 2001) Although genome analyses have suggested parallels between basis of asymmetry in base composition between leading and archaeal and eukaryotic replication systems, little is known about lagging strands (10, 11). In Pyrococcus abyssi, the location of the the DNA replication mechanism in Archaea. By two-dimensional predicted origin coincides with an early replicating chromosomal gel electrophoreses we positioned a replication origin (oriC) within segment of 80 kb identified by radioactive labeling of chromo- 1 kb in the chromosomal DNA of Pyrococcus abyssi, an anaerobic somal DNA in cultures released from a replication block (12). hyperthermophile, and demonstrated that the oriC is physically Our in silico and labeling data also allowed us to conclude that linked to the cdc6 gene. Our chromatin immunoprecipitation as- the hyperthermophilic archaeon P. abyssi uses a single bidirec- says indicated that P. abyssi Cdc6 and minichromosome mainte- tional origin to replicate its genome. We proposed that this origin nance (MCM) proteins bind preferentially to the oriC region in the would correspond to the long intergenic region conserved in all exponentially growing cells. Whereas the oriC association of MCM three known Pyrococcus sp. -

CUL4B Promotes Replication Licensing by Up-Regulating the CDK2–CDC6 Cascade
JCB: Article CUL4B promotes replication licensing by up-regulating the CDK2–CDC6 cascade Yongxin Zou,1,2 Jun Mi,1 Wenxing Wang,1 Juanjuan Lu,1 Wei Zhao,1 Zhaojian Liu,1 Huili Hu,1 Yang Yang,1 Xiaoxing Gao,1 Baichun Jiang,1 Changshun Shao,1 and Yaoqin Gong1 1Ministry of Education Key Laboratory of Experimental Teratology and Institute of Molecular Medicine and Genetics, Shandong University School of Medicine, Jinan, Shandong 250012, China 2Section of Biochemistry and Cell Biology, Division of Life Science, and Center for Cancer Research, Hong Kong University of Science and Technology, Clear Water Bay, Kowloon, Hong Kong, China ullin-RING ubiquitin ligases (CRLs) participate in loading of MCM2 to chromatin. The positive regulation of the regulation of diverse cellular processes in CDC6 by CUL4B depends on CDK2, which phosphory- C cluding cell cycle progression. Mutations in the lates CDC6, protecting it from APCCDH1-mediated degra- X-linked CUL4B, a member of the cullin family, cause mental dation. Thus, aside being required for cell cycle reentry retardation and other developmental abnormalities in from quiescence, CDK2 also contributes to pre-replication humans. Cells that are deficient in CUL4B are severely complex assembly in G1 phase of cycling cells. Interest- selected against in vivo in heterozygotes. Here we report ingly, the up-regulation of CDK2 by CUL4B is achieved a role of CUL4B in the regulation of replication licensing. via the repression of miR-372 and miR-373, which target Strikingly, CDC6, the licensing factor in replication, was CDK2. Our findings thus establish a CUL4B–CDK2–CDC6 positively regulated by CUL4B and contributed to the cascade in the regulation of DNA replication licensing. -

Control of Eukaryotic DNA Replication Initiation—Mechanisms to Ensure Smooth Transitions
G C A T T A C G G C A T genes Review Control of Eukaryotic DNA Replication Initiation—Mechanisms to Ensure Smooth Transitions Karl-Uwe Reusswig and Boris Pfander * Max Planck Institute of Biochemistry, DNA Replication and Genome Integrity, 82152 Martinsried, Germany; [email protected] * Correspondence: [email protected] Received: 31 December 2018; Accepted: 25 January 2019; Published: 29 January 2019 Abstract: DNA replication differs from most other processes in biology in that any error will irreversibly change the nature of the cellular progeny. DNA replication initiation, therefore, is exquisitely controlled. Deregulation of this control can result in over-replication characterized by repeated initiation events at the same replication origin. Over-replication induces DNA damage and causes genomic instability. The principal mechanism counteracting over-replication in eukaryotes is a division of replication initiation into two steps—licensing and firing—which are temporally separated and occur at distinct cell cycle phases. Here, we review this temporal replication control with a specific focus on mechanisms ensuring the faultless transition between licensing and firing phases. Keywords: DNA replication; DNA replication initiation; cell cycle; post-translational protein modification; protein degradation; cell cycle transitions 1. Introduction DNA replication control occurs with exceptional accuracy to keep genetic information stable over as many as 1016 cell divisions (estimations based on [1]) during, for example, an average human lifespan. A fundamental part of the DNA replication control system is dedicated to ensure that the genome is replicated exactly once per cell cycle. If this control falters, deregulated replication initiation occurs, which leads to parts of the genome becoming replicated more than once per cell cycle (reviewed in [2–4]). -

Preventing Rereplication Via Multiple Mechanisms in Eukaryotic Cells
Downloaded from genesdev.cshlp.org on September 29, 2021 - Published by Cold Spring Harbor Laboratory Press REVIEW Strength in numbers: preventing rereplication via multiple mechanisms in eukaryotic cells Emily E. Arias1 and Johannes C. Walter2 Department of Biological Chemistry and Molecular Pharmacology, Harvard Medical School, 240 Longwood Avenue, Boston, Massachusetts 02115, USA In eukaryotic cells, prereplication complexes (pre-RCs) ity. Thus, the ability of cells to restrict DNA replication are assembled on chromatin in the G1 phase, rendering to a single round per cell cycle is a fundamental require- origins of DNA replication competent to initiate DNA ment of cell proliferation and long-term survival. synthesis. When DNA replication commences in S phase, pre-RCs are disassembled, and multiple initia- The two-state model for cell cycle regulation tions from the same origin do not occur because new of DNA replication rounds of pre-RC assembly are inhibited. In most experi- Early insights into the regulation of eukaryotic DNA mental organisms, multiple mechanisms that prevent replication came from cell fusion experiments (Rao and pre-RC assembly have now been identified, and rerepli- Johnson 1970), which showed that union of an S-phase cation within the same cell cycle can be induced through cell with a G1 cell accelerates the rate at which the latter defined perturbations of these mechanisms. This review enters S phase. In contrast, G2 cells are refractory to this summarizes the diverse array of inhibitory pathways stimulation. These results suggested that the initiation used by different organisms to prevent pre-RC assembly, of DNA synthesis requires a positive, diffusible S-phase- and focuses on the challenge of understanding how in promoting activity, and that G1 but not G2-phase cells any one cell type, various mechanisms cooperate to are competent to respond to this signal. -
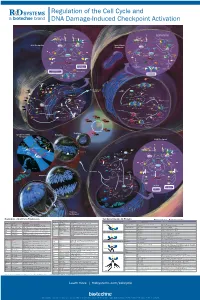
Regulation of the Cell Cycle and DNA Damage-Induced Checkpoint Activation
RnDSy-lu-2945 Regulation of the Cell Cycle and DNA Damage-Induced Checkpoint Activation IR UV IR Stalled Replication Forks/ BRCA1 Rad50 Long Stretches of ss-DNA Rad50 Mre11 BRCA1 Nbs1 Rad9-Rad1-Hus1 Mre11 RPA MDC1 γ-H2AX DNA Pol α/Primase RFC2-5 MDC1 Nbs1 53BP1 MCM2-7 53BP1 γ-H2AX Rad17 Claspin MCM10 Rad9-Rad1-Hus1 TopBP1 CDC45 G1/S Checkpoint Intra-S-Phase RFC2-5 ATM ATR TopBP1 Rad17 ATRIP ATM Checkpoint Claspin Chk2 Chk1 Chk2 Chk1 ATR Rad50 ATRIP Mre11 FANCD2 Ubiquitin MDM2 MDM2 Nbs1 CDC25A Rad50 Mre11 BRCA1 Ub-mediated Phosphatase p53 CDC25A Ubiquitin p53 FANCD2 Phosphatase Degradation Nbs1 p53 p53 CDK2 p21 p21 BRCA1 Ub-mediated SMC1 Degradation Cyclin E/A SMC1 CDK2 Slow S Phase CDC45 Progression p21 DNA Pol α/Primase Slow S Phase p21 Cyclin E Progression Maintenance of Inhibition of New CDC6 CDT1 CDC45 G1/S Arrest Origin Firing ORC MCM2-7 MCM2-7 Recovery of Stalled Replication Forks Inhibition of MCM10 MCM10 Replication Origin Firing DNA Pol α/Primase ORI CDC6 CDT1 MCM2-7 ORC S Phase Delay MCM2-7 MCM10 MCM10 ORI Geminin EGF EGF R GAB-1 CDC6 CDT1 ORC MCM2-7 PI 3-Kinase p70 S6K MCM2-7 S6 Protein Translation Pre-RC (G1) GAB-2 MCM10 GSK-3 TSC1/2 MCM10 ORI PIP2 TOR Promotes Replication CAK EGF Origin Firing Origin PIP3 Activation CDK2 EGF R Akt CDC25A PDK-1 Phosphatase Cyclin E/A SHIP CIP/KIP (p21, p27, p57) (Active) PLCγ PP2A (Active) PTEN CDC45 PIP2 CAK Unwinding RPA CDC7 CDK2 IP3 DAG (Active) Positive DBF4 α Feedback CDC25A DNA Pol /Primase Cyclin E Loop Phosphatase PKC ORC RAS CDK4/6 CDK2 (Active) Cyclin E MCM10 CDC45 RPA IP Receptor -

A Free-Living Protist That Lacks Canonical Eukaryotic DNA Replication and Segregation Systems
bioRxiv preprint doi: https://doi.org/10.1101/2021.03.14.435266; this version posted March 15, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 A free-living protist that lacks canonical eukaryotic DNA replication and segregation systems 2 Dayana E. Salas-Leiva1, Eelco C. Tromer2,3, Bruce A. Curtis1, Jon Jerlström-Hultqvist1, Martin 3 Kolisko4, Zhenzhen Yi5, Joan S. Salas-Leiva6, Lucie Gallot-Lavallée1, Geert J. P. L. Kops3, John M. 4 Archibald1, Alastair G. B. Simpson7 and Andrew J. Roger1* 5 1Centre for Comparative Genomics and Evolutionary Bioinformatics (CGEB), Department of 6 Biochemistry and Molecular Biology, Dalhousie University, Halifax, NS, Canada, B3H 4R2 2 7 Department of Biochemistry, University of Cambridge, Cambridge, United Kingdom 8 3Oncode Institute, Hubrecht Institute – KNAW (Royal Netherlands Academy of Arts and Sciences) 9 and University Medical Centre Utrecht, Utrecht, The Netherlands 10 4Institute of Parasitology Biology Centre, Czech Acad. Sci, České Budějovice, Czech Republic 11 5Guangzhou Key Laboratory of Subtropical Biodiversity and Biomonitoring, School of Life Science, 12 South China Normal University, Guangzhou 510631, China 13 6CONACyT-Centro de Investigación en Materiales Avanzados, Departamento de medio ambiente y 14 energía, Miguel de Cervantes 120, Complejo Industrial Chihuahua, 31136 Chihuahua, Chih., México 15 7Centre for Comparative Genomics and Evolutionary Bioinformatics (CGEB), Department of 16 Biology, Dalhousie University, Halifax, NS, Canada, B3H 4R2 17 *corresponding author: [email protected] 18 D.E.S-L ORCID iD: 0000-0003-2356-3351 19 E.C.T.