First Detailed Description of the Nervous System of the Most Complex Lophophore and Evolution of Brachiopoda
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Zootaxa, Mollusca, Goniodorididae, Okenia
ZOOTAXA 695 Further species of the opisthobranch genus Okenia (Nudibranchia: Goniodorididae) from the Indo-West Pacific W.B. RUDMAN Magnolia Press Auckland, New Zealand W.B. RUDMAN Further species of the opisthobranch genus Okenia (Nudibranchia: Goniodorididae) from the Indo-West Pacific (Zootaxa 695) 70 pp.; 30 cm. 25 October 2004 ISBN 1-877354-68-6 (Paperback) ISBN 1-877354-69-4 (Online edition) FIRST PUBLISHED IN 2004 BY Magnolia Press P.O. Box 41383 Auckland 1030 New Zealand e-mail: [email protected] http://www.mapress.com/zootaxa/ © 2004 Magnolia Press All rights reserved. No part of this publication may be reproduced, stored, transmitted or disseminated, in any form, or by any means, without prior written permission from the publisher, to whom all requests to reproduce copyright material should be directed in writing. This authorization does not extend to any other kind of copying, by any means, in any form, and for any purpose other than private research use. ISSN 1175-5326 (Print edition) ISSN 1175-5334 (Online edition) Zootaxa 695: 1–70 (2004) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA 695 Copyright © 2004 Magnolia Press ISSN 1175-5334 (online edition) Further species of the opisthobranch genus Okenia (Nudibranchia: Goniodorididae) from the Indo-West Pacific W.B. RUDMAN The Australian Museum, 6 College St., Sydney, NSW 2010, Australia. E-mail: [email protected]. Table of Contents Abstract . 3 Introduction. 4 Materials and Methods . 5 Descriptions . 5 Okenia Menke, 1830. 5 Okenia echinata Baba, 1949. 6 Okenia purpurata sp. nov. 9 Okenia vena sp. nov.. 13 Okenia virginiae Gosliner, 2004. -

Review Sheet for Lecture 15: Life in the Benthic Realm
Lecture 15: Life in the Benthic Realm Terminology Planktonic, nektonic, benthic, epifaunal, infaunal, sessile, mobile, bathyal, abyssal, abyssopelagic, hadopelagic, supratidal, intertidal, subtidal, spicules, spongin, radial symmetry, encruster, epidermal cell, porocyte, sclerocyte, amoebocyte, mesohyl, choanocyte, polyp, medusa, colonial, solitary, aragonite, octocoral, gorgonin, cnidocyst, nematocyst, zooxanthellae, hematypic, coral bleaching, lophophore, zoid, zooecium, zooaria, polymorphism, pedicle, helically coiled shell, radially coiled shell, exoskeleton, endoskeleton Dates: none Review Questions: Be familiar with the life and feeding modes of all of the invertebrate groups we discussed and their major morphologies, behaviors, life strategies etc. Why are so many sessile invertebrates radially symmetric? Explain the three different wall types found in sponges, what’s the point? Discuss the different roles of the different sponge cells How do hematypic corals contribute to the creation of different nearshore environments and conditions? Describe the community created by an innkeeper worm How does the skeleton of a brachiopod differ from that of a clam? How do the shells of clams, snails, and nautiloids differ? Classification: Phylum Porifera Class Demospongiae (soft sponges) Class Calcarea (calcareous sponges) Class Hexactinellida (glass sponges) Phylum Cnidaria Class Anthozoa -stony corals -sea fans -soft corals -sea anemones -sea pens Phylum Annelida (worms) -bristleworms -innkeeper worm -lugworms -spaghetti worm -feather duster -catworm Phylum Bryozoa (moss-animals) Phylum Mollusca -Class Bivalvia (clams) -Class Gastropoda (snails and slugs) -Class Cephalopoda (squid, octopus, nautiloid) -Class Scaphopoda (tusk shells) -Class Polyplacophora (chitons) . -

DEEP SEA LEBANON RESULTS of the 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project
DEEP SEA LEBANON RESULTS OF THE 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project March 2018 DEEP SEA LEBANON RESULTS OF THE 2016 EXPEDITION EXPLORING SUBMARINE CANYONS Towards Deep-Sea Conservation in Lebanon Project Citation: Aguilar, R., García, S., Perry, A.L., Alvarez, H., Blanco, J., Bitar, G. 2018. 2016 Deep-sea Lebanon Expedition: Exploring Submarine Canyons. Oceana, Madrid. 94 p. DOI: 10.31230/osf.io/34cb9 Based on an official request from Lebanon’s Ministry of Environment back in 2013, Oceana has planned and carried out an expedition to survey Lebanese deep-sea canyons and escarpments. Cover: Cerianthus membranaceus © OCEANA All photos are © OCEANA Index 06 Introduction 11 Methods 16 Results 44 Areas 12 Rov surveys 16 Habitat types 44 Tarablus/Batroun 14 Infaunal surveys 16 Coralligenous habitat 44 Jounieh 14 Oceanographic and rhodolith/maërl 45 St. George beds measurements 46 Beirut 19 Sandy bottoms 15 Data analyses 46 Sayniq 15 Collaborations 20 Sandy-muddy bottoms 20 Rocky bottoms 22 Canyon heads 22 Bathyal muds 24 Species 27 Fishes 29 Crustaceans 30 Echinoderms 31 Cnidarians 36 Sponges 38 Molluscs 40 Bryozoans 40 Brachiopods 42 Tunicates 42 Annelids 42 Foraminifera 42 Algae | Deep sea Lebanon OCEANA 47 Human 50 Discussion and 68 Annex 1 85 Annex 2 impacts conclusions 68 Table A1. List of 85 Methodology for 47 Marine litter 51 Main expedition species identified assesing relative 49 Fisheries findings 84 Table A2. List conservation interest of 49 Other observations 52 Key community of threatened types and their species identified survey areas ecological importanc 84 Figure A1. -

Treatise on Invertebrate Paleontology
PART H, Revised BRACHIOPODA VOLUMES 2 & 3: Linguliformea, Craniiformea, and Rhynchonelliformea (part) ALWYN WILLIAMS, S. J. CARLSON, C. H. C. BRUNTON, L. E. HOLMER, L. E. POPOV, MICHAL MERGL, J. R. LAURIE, M. G. BASSETT, L. R. M. COCKS, RONG JIA-YU, S. S. LAZAREV, R. E. GRANT, P. R. RACHEBOEUF, JIN YU-GAN, B. R. WARDLAW, D. A. T. HARPER, A. D. WRIGHT, and MADIS RUBEL CONTENTS INFORMATION ON TREATISE VOLUMES ...................................................................................... x EDITORIAL PREFACE .............................................................................................................. xi STRATIGRAPHIC DIVISIONS .................................................................................................. xxiv COORDINATING AUTHOR'S PREFACE (Alwyn Williams) ........................................................ xxv BRACHIOPOD CLASSIFICATION (Alwyn Williams, Sandra J. Carlson, and C. Howard C. Brunton) .................................. 1 Historical Review .............................................................................................................. 1 Basis for Classification ....................................................................................................... 5 Methods.......................................................................................................................... 5 Genealogies ....................................................................................................................... 6 Recent Brachiopods ....................................................................................................... -
Bryozoa of the Caspian Sea
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/339363855 Bryozoa of the Caspian Sea Article in Inland Water Biology · January 2020 DOI: 10.1134/S199508292001006X CITATIONS READS 0 90 1 author: Valentina Ivanovna Gontar Russian Academy of Sciences 58 PUBLICATIONS 101 CITATIONS SEE PROFILE Some of the authors of this publication are also working on these related projects: Freshwater Bryozoa View project Evolution of spreading of marine invertebrates in the Northern Hemisphere View project All content following this page was uploaded by Valentina Ivanovna Gontar on 19 February 2020. The user has requested enhancement of the downloaded file. ISSN 1995-0829, Inland Water Biology, 2020, Vol. 13, No. 1, pp. 1–13. © Pleiades Publishing, Ltd., 2020. Russian Text © The Author(s), 2020, published in Biologiya Vnutrennykh Vod, 2020, No. 1, pp. 3–16. AQUATIC FLORA AND FAUNA Bryozoa of the Caspian Sea V. I. Gontar* Institute of Zoology, Russian Academy of Sciences, St. Petersburg, Russia *e-mail: [email protected] Received April 24, 2017; revised September 18, 2018; accepted November 27, 2018 Abstract—Five bryozoan species of the class Gymnolaemata and a single Plumatella emarginata species of the class Phylactolaemata are found in the Caspian Sea. The class Gymnolaemata is represented by bryozoans of the orders Ctenostomatida (Amathia caspia, Paludicella articulata, and Victorella pavida) and Cheilostoma- tida (Conopeum grimmi and Lapidosella ostroumovi). Two species (Conopeum grimmi and Amatia caspia) are Caspian endemics. Lapidosella ostroumovi was identified in the Caspian Sea for the first time. The systematic position, illustrated morphological descriptions, and features of ecology of the species identified are pre- sented. -

Invertebrates Invertebrates: • Are Animals Without Backbones • Represent 95% of the Animal Kingdom Animal Diversity Morphological Vs
Invertebrates Invertebrates: • Are animals without backbones • Represent 95% of the animal kingdom Animal Diversity Morphological vs. Molecular Character Phylogeny? A tree is a hypothesis supported or not supported by evidence. Groupings change as new evidence become available. Sponges - Porifera Natural Bath Sponges – over-collected, now uncommon Sponges • Perhaps oldest animal phylum (Ctenphora possibly older) • may represent several old phyla, some now extinct ----------------Ctenophora? Sponges - Porifera • Mostly marine • Sessile animals • Lack true tissues; • Have only a few cell types, cells kind of independent • Most have no symmetry • Body resembles a sac perforated with holes, system of canals. • Strengthened by fibers of spongin, spicules Sponges have a variety of shapes Sponges Pores Choanocyte Amoebocyte (feeding cell) Skeletal Water fiber flow Central cavity Flagella Choanocyte in contact with an amoebocyte Sponges - Porifera • Sessile filter feeder • No mouth • Sac-like body, perforated by pores. • Interior lined by flagellated cells (choanocytes). Flagellated collar cells generate a current, draw water through the walls of the sponge where food is collected. • Amoeboid cells move around in the mesophyll and distribute food. Sponges - Porifera Grantia x.s. Sponge Reproduction Asexual reproduction • Fragmentation or by budding. • Sponges are capable of regeneration, growth of a whole from a small part. Sexual reproduction • Hermaphrodites, produce both eggs and sperm • Eggs and sperm released into the central cavity • Produces -
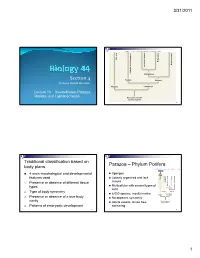
S I Section 4
3/31/2011 Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Porifera Ecdysozoa Deuterostomia Lophotrochozoa Cnidaria and Ctenophora Cnidaria and Protostomia SSiection 4 Radiata Bilateria Professor Donald McFarlane Parazoa Eumetazoa Lecture 13 Invertebrates: Parazoa, Radiata, and Lophotrochozoa Ancestral colonial choanoflagellate 2 Traditional classification based on Parazoa – Phylum Porifera body plans Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Parazoa 4 main morphological and developmental Sponges features used Loosely organized and lack Porifera Ecdysozoa Cnidaria and Ctenophora tissues euterostomia photrochozoa D 1. o Presence or absence of different tissue L Multicellular with several types of types Protostomia cells 2. Type of body symmetry Radiata Bilateria 8,000 species, mostly marine Parazoa Eumetazoa 3. Presence or absence of a true body No apparent symmetry cavity Ancestral colonial Adults sessile, larvae free- choanoflagellate 4. Patterns of embryonic development swimming 3 4 1 3/31/2011 Water drawn through pores (ostia) into spongocoel Flows out through osculum Reproduce Choanocytes line spongocoel Sexually Most hermapppgggphrodites producing eggs and sperm Trap and eat small particles and plankton Gametes are derived from amoebocytes or Mesohyl between choanocytes and choanocytes epithelial cells Asexually Amoebocytes absorb food from choanocytes, Small fragment or bud may detach and form a new digest it, and carry -

Practical Paleoecology GEO 342
Practical Paleoecology GEO 342 Compiled By: Dr. Osama Attia Compiled By Dr. Osama Attia 1 Phylum Brachiopoda Brachiopods or lamp shells Name: Name means "arm" (brachio) + "foot" (pod). Main characteristics: - Bivalved (two shells), each with bilateral symmetry. - The plane of symmetry passes through the center of each shell or valve. - The two valves differ in size and shape in most. Sometimes the larger valve will have an opening near the hinge line through which the pedicle extended in life. - Soft parts include a lophophore consisting of coiled tentacles with cilia. The lophophore circulates water between the two valves, distributing oxygen and flushing out carbon dioxide. Water movements caused by the lophophore also transport food particles toward the mouth. Geologic range: Lower Cambrian to Recent. Very abundant during the Paleozoic. A few species (belonging to only three families) remain today. Mode of life: Inhabitants of shallow marine environments; they generally live attached in a fixed position on the seafloor. Inarticulate brachiopods are known to live in burrows in the sediment. Brachiopods are filter feeders. Compiled By Dr. Osama Attia 2 Living positions of brachiopods. A = Articulate brachiopod attached to the seafloor by its pedicle. B = Interior of brachiopod valve showing lophophore. C = Inarticulate brachiopod, Lingula, which lives within a tube or burrow in seafloor sediment. A. CLASS INARTICULATA – The Inarticulate Brachiopods Primitive brachiopods with phosphatic or chitinous valves; no hinge. Spoon-shaped valves held together with muscles and soft parts. Lingula is a well known inarticulate brachiopod. Geologic range: Lower Cambrian to Recent Compiled By Dr. Osama Attia 3 Fossil inarticulate brachiopod from the Cambrian. -

BRIOZOÁRIOS DO FOULING: Avaliação Das Espécies Exóticas E Invasoras Do Brasil
UNIVERSIDADE FEDERAL DE PERNAMBUCO CENTRO DE BIOCIÊNCIAS DEPARTAMENTO DE ZOOLOGIA PROGRAMA DE PÓS-GRADUAÇÃO EM BIOLOGIA ANIMAL ADÉLIA ALLIZ MIRANDA BRIOZOÁRIOS DO FOULING: avaliação das espécies exóticas e invasoras do Brasil RECIFE 2018 ADÉLIA ALLIZ MIRANDA BRIOZOÁRIOS DO FOULING: avaliação das espécies exóticas e invasoras do Brasil Dissertação apresentada ao Programa de Pós-Graduação em Biologia Animal da Universidade Federal de Pernambuco como requisito parcial para obtenção do título de Mestre em Biologia Animal. Orientador: Prof. Dr. Leandro Manzoni Vieira RECIFE 2018 Catalogação na fonte: Bibliotecário: Bruno Márcio Gouveia - CRB-4/1788 Miranda, Adélia Alliz Briozoários do fouling : avaliação das espécies exóticas invasoras do Brasil / Adélia Alliz Miranda. – 2018. 121 f. : il. Orientador: Prof. Dr. Leandro Manzoni Vieira. Dissertação (mestrado) – Universidade Federal de Pernambuco. Centro de Biociências. Programa de Pós-graduação em Biologia Animal, Recife, 2018. Inclui referências e apêndices. 1. Animais marinhos. 2. Invertebrados marinhos. I. Vieira, Leandro Manzoni (Orientador). II. Título. 592 CDD (22.ed.) UFPE/CB – 2018 - 373 1 ADÉLIA ALLIZ MIRANDA BRIOZOÁRIOS DO FOULING: avaliação das espécies exóticas e invasoras do Brasil Dissertação apresentada ao Programa de Pós- Graduação em Biologia Animal da Universidade Federal de Pernambuco como requisito parcial para obtenção do título de Mestre em Biologia Animal. Aprovada em: 19/07/2018 BANCA EXAMINADORA _______________________________________________________________ Prof. Dr. André Morgado Esteves Universidade Federal de Pernambuco ______________________________________________________________ Prof. Dr. Rosana Moreira da Rocha Universidade Federal do Paraná ______________________________________________________ Prof. Dr. Alvaro Esteves Migotto Universidade de São Paulo 2 AGRADECIMENTOS Agradeço em primeiro lugar aos meus pais, Thereza e Antônio, pois tudo que sou devo a eles. -

Biodiversity Patterns Across the Late Paleozoic Ice Age
Palaeontologia Electronica palaeo-electronica.org Biodiversity patterns across the Late Paleozoic Ice Age Barbara Seuss, Vanessa Julie Roden, and Ádám T. Kocsis ABSTRACT The Late Palaeozoic Ice Age (LPIA, Famennian to Wuchiapingian) witnessed two transitions between ice- and greenhouse conditions. These alternations led to drastic alterations in the marine system (e.g., sea-level, habitat size, sea-surface temperature) forcing faunal changes. To reassess the response of the global marine fauna, we ana- lyze diversity dynamics of brachiopod, bivalve, and gastropod taxa throughout the LPIA using data from the Paleobiology Database. Diversity dynamics were assessed regarding environmental affinities of these clades. Our analyses indicate that during the LPIA more taxa had an affinity towards siliciclastic than towards carbonate environ- ments. Deep-water and reefal habitats were more favored while grain size was less determining. In individual stages of the LPIA, the clades show rather constant affinities towards an environment. Those bivalves and brachiopods with an affinity differ in their habitat preferences, indicating that there might have been little competition among these two clades. Origination and extinction rates are similar during the main phase of the LPIA, whether environmental affinities are considered or not. This underlines that the LPIA marine fauna was well adapted and capable of reacting to changing environ- mental and climatic conditions. Since patterns of faunal change are similar in different environments, our study implies that the changes in faunal composition (e.g., diversity loss during the LPIA; strong increase of brachiopod diversity during the Permian) were influenced by the habitat to only a minor degree but most likely by yet unknown abiotic factors. -

On the History of the Names Lingula, Anatina, and on the Confusion of the Forms Assigned Them Among the Brachiopoda Christian Emig
On the history of the names Lingula, anatina, and on the confusion of the forms assigned them among the Brachiopoda Christian Emig To cite this version: Christian Emig. On the history of the names Lingula, anatina, and on the confusion of the forms assigned them among the Brachiopoda. Carnets de Geologie, Carnets de Geologie, 2008, CG2008 (A08), pp.1-13. <hal-00346356> HAL Id: hal-00346356 https://hal.archives-ouvertes.fr/hal-00346356 Submitted on 11 Dec 2008 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Carnets de Géologie / Notebooks on Geology - Article 2008/08 (CG2008_A08) On the history of the names Lingula, anatina, and on the confusion of the forms assigned them among the Brachiopoda 1 Christian C. EMIG Abstract: The first descriptions of Lingula were made from then extant specimens by three famous French scientists: BRUGUIÈRE, CUVIER, and LAMARCK. The genus Lingula was created in 1791 (not 1797) by BRUGUIÈRE and in 1801 LAMARCK named the first species L. anatina, which was then studied by CUVIER (1802). In 1812 the first fossil lingulids were discovered in the Mesozoic and Palaeozoic strata of the U.K. -

Molecular Evidence That Phoronids Are a Subtaxon of Brachiopods
Cohen, B. L. and Weydmann, A. (2005) Molecular evidence that phoronids are a subtaxon of brachiopods (Brachiopoda: Phoronata) and that genetic divergence of metazoan phyla began long before the early Cambrian. Organisms Diversity and Evolution 5(4):pp. 253-273. http://eprints.gla.ac.uk/2944/ Glasgow ePrints Service http://eprints.gla.ac.uk ARTICLE IN PRESS Organisms, Diversity & Evolution 5 (2005) 253–273 www.elsevier.de/ode from: Organisms, Diversity & Evolution (2005) 5: 253-273 Molecular evidence that phoronids are a subtaxon of brachiopods (Brachiopoda: Phoronata) and that genetic divergence of metazoan phyla began long before the early Cambrian Bernard L. Cohena,Ã, Agata Weydmannb aIBLS, Division of Molecular Genetics, University of Glasgow, Pontecorvo Building, 56 Dumbarton Road, Glasgow, G11 6NU, Scotland, UK bInstitute of Oceanology, Polish Academy of Sciences, Powstancow Warszawy St., 55, 81-712-Sopot, Poland Received 4 October 2004; accepted 22 December 2004 Abstract Concatenated SSU (18S) and partial LSU (28S) sequences (2 kb) from 12 ingroup taxa, comprising 2 phoronids, 2 members of each of the craniid, discinid, and lingulid inarticulate brachiopod lineages, and 4 rhynchonellate, articulate brachiopods (2 rhynchonellides, 1 terebratulide and 1 terebratellide) were aligned with homologous sequences from 6 protostome, deuterostome and sponge outgroups (3964 sites). Regions of potentially ambiguous alignment were removed, and the resulting data (3275 sites, of which 377 were parsimony-informative and 635 variable) were analysed by parsimony, and by maximum and Bayesian likelihood using objectively selected models. There was no base composition heterogeneity. Relative rate tests led to the exclusion (from most analyses) of the more distant outgroups, with retention of the closer pectinid and polyplacophoran (chiton).