Purinergic Regulation of Neutrophil Function
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Purinergic Receptor P2Y, G-Protein Coupled, 2 (P2RY2) Gene Associated with Essential Hypertension in Japanese Men
Journal of Human Hypertension (2010) 24, 327–335 & 2010 Macmillan Publishers Limited All rights reserved 0950-9240/10 $32.00 www.nature.com/jhh ORIGINAL ARTICLE The purinergic receptor P2Y, G-protein coupled, 2 (P2RY2) gene associated with essential hypertension in Japanese men Z Wang1,2, T Nakayama1,3, N Sato1, Y Izumi3, Y Kasamaki4, M Ohta4, M Soma5, N Aoi1, Y Ozawa4 andYMa2 1Division of Laboratory Medicine, Department of Pathology and Microbiology, Nihon University School of Medicine, Tokyo, Japan; 2Department of Cardiovascular Medicine, the First Affiliated Hospital of Xinjiang Medical University, Urumqi, Xinjiang, PR China; 3Division of Nephrology, Hypertension and Endocrinology, Department of Medicine, Nihon University School of Medicine, Tokyo, Japan; 4Division of Cardiovascular Medicine, Department of Medicine, Nihon University School of Medicine, Tokyo, Japan and 5Division of General Medicine, Department of Medicine, Nihon University School of Medicine, Tokyo, Japan P2RY2 has an important function in the regulation of respectively). Logistic regression showed that for blood pressure by activating adenosine triphosphate the total and men groups, the TG þ TT genotype of (ATP). The aim of this study was to investigate the asso- rs4944831 was more prevalent in EH patients than in the ciation between the human P2RY2 gene and essential controls (P ¼ 0.026 and 0.011, respectively). For men, the hypertension (EH) through a haplotype-based case– overall distribution of the haplotype (SNP2-SNP4-SNP5) control study that included two gender groups. The 273 was significantly different between the EH patients EH patients and 255 age-matched controls were geno- and the controls (P ¼ 0.006). As compared with controls, typed for five single-nucleotide polymorphisms (SNPs) the frequency of the T-A-G haplotype was significantly of the human P2RY2 gene (rs4944831, rs1783596, higher, whereas the T-C-G haplotype was significan- rs4944832, rs4382936 and rs10898909). -

Activation of Hypermethylated P2RY1 Mitigates Gastric Cancer by Promoting Apoptosis and Inhibiting Proliferation
Activation of hypermethylated P2RY1 mitigates gastric cancer by promoting apoptosis and inhibiting proliferation Yinggang Hua Xiamen University Medical College Long Li Xiamen University Medical College Liangliang Cai Zhongshan Hospital Xiamen University Guoyan Liu ( [email protected] ) Zhongshan Hospital Xiamen University Research Article Keywords: Diffuse type gastric cancer, DNA methylation 450K array, P2RY1 receptor, ERK signal pathway, Tumor suppressor gene Posted Date: July 26th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-351723/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/16 Abstract P2RY1 receptor is known to cause cancer by activating the ERK signal pathway, its DNA methylation status or even the corresponding regulatory mechanism remains unknown. In this study, DNA methylation chip was used to prole the genome-wide DNA methylation level in gastric cancer tissues. Proliferation and apoptosis of the SGC7901 gastric cancer cell line were determined after treatment with a selective P2RY1 receptor agonist, MRS2365. The promoter region of P2RY1 was found to be highly methylated with 4 hypermethylated sites (|Δβ value| >0.2) in diffuse gastric cancer and then were validated by bioinformatic analysis in TCGA database. Analysis of MRS2365-treated cells by annexin-V/PI staining and Caspase-3 activity assays indicated the induction of apoptosis in SGC7901 cells. P2RY1 receptor activation in human SGC7901 gastric cancer cells via the MRS2365 agonist induced apoptosis and reduced cell growth. High DNA methylation in the promoter region of P2RY1 may have contributed to the reduced expression of P2RY1’s mRNA, which is likely responsible for the “aggressive” nature of the diffuse type gastric cancer. -

Bioinformatics Unmasks the Maneuverers of Pain Pathways In
www.nature.com/scientificreports OPEN Bioinformatics Unmasks the Maneuverers of Pain Pathways in Acute Kidney Injury Received: 4 March 2019 Aprajita Gupta 1, Sanjeev Puri 2 & Veena Puri 1 Accepted: 31 July 2019 Acute Kidney injury (AKI) is one of the leading health concerns resulting in accumulation of nitrogenous Published: xx xx xxxx as well as non-nitrogenous wastes in body and characterised by a rapid deterioration in kidney functions. Besides the major toll from the primary insult in the kidney, consequential extra-renal secondary insults endowed with the pathways of infammatory milieu often complicates the disease outcome. Some of the known symptoms of AKI leading to clinical reporting are fatigue, loss of appetite, headache, nausea, vomiting, and pain in the fanks, wherein proinfammatory cytokines have been strongly implicated in pathogenesis of AKI and neuro-infammation. Taking in account these clues, we have tried to decode the neuro-infammation and pain perception phenomenon during the progression of AKI using the pathway integration and biological network strategies. The pathways and networks were generated using bioinformatics software viz. PANTHER, Genomatix and PathVisio to establish the relationship between immune and neuro related pathway in AKI. These observations envisage a neurol-renal axis that is predicted to involve calcium channels in neuro-infammatory pathway of AKI. These observations, thus, pave a way for a new paradigm in understanding the interplay of neuro- immunological signalling in AKI. Acute kidney injury (AKI) is a clinical event associated with a rapid loss of kidney function, leading to high mor- bidity and mortality1. Every year about 2 million people die from AKI due to late detection of disease or paucity of efective therapeutic interventions2. -

Initial, Transient, and Specific Interaction Between G Protein
Sato T. et al. Medical Research Archives, vol. 6, issue 9, September 2018 Page 1 of 25 ARTICLE Initial, transient, and specific interaction between G protein-coupled receptor and target G protein in parallel signal processing: a case of olfactory discrimination of cancer-induced odors Takaaki Sato1, Mutsumi Matsukawa2, Yoichi Mizutani3, Toshio Iijima4, Hiroyoshi Matsumura5 Authors’ affiliations: 1 Biomedical Research Institute, National Institute of Advanced Industrial Science and Technology, Osaka, Japan 2 Division of Anatomical Science, Department of Functional Morphology, Nihon University School of Medicine, Tokyo, Japan 3 Department of Medical Engineering, Faculty of Health Science, Aino University, Osaka, Japan 4 Graduate School of Life Sciences, Tohoku University, Sendai, Japan 5 College of Life Sciences, Ritsumeikan University, Kusatsu, Japan * Corresponding author: Takaaki Sato, Biomedical Research Institute, National Institute of Ad- vanced Industrial Science and Technology, 1-8-31 Midorioka, Ikeda, Osaka 563-8577, Japan, E-mail: [email protected] Abstract: G protein-coupled receptors (GPCRs) detect and distinguish between various subtypes of extracellular sig- nals, such as neurotransmitters, hormones, light, and odorous chemicals. As determinants for robust and appropriate cellular responses, common and unique features of interactions between GPCRs and their target G proteins provide insights into structure-based drug design for treatment of GPCR-related diseases. Re- cently, we found that the hydrophobic core buried between GPCR helix 8 and TM1–2 is essential for acti- vation of both specific and nonspecific G proteins. Furthermore, the 2nd residue of helix 8 is responsible for initial, transient, and specific interaction with a target G protein. Analysis of human and murine olfactory receptors (ORs) and other class-A GPCRs revealed that several amino acids, such as Glu, Gln, and Asp, are conserved at this position. -

The G Protein-Coupled Receptor Heterodimer Network (GPCR-Hetnet) and Its Hub Components
Int. J. Mol. Sci. 2014, 15, 8570-8590; doi:10.3390/ijms15058570 OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Article The G Protein-Coupled Receptor Heterodimer Network (GPCR-HetNet) and Its Hub Components Dasiel O. Borroto-Escuela 1,†,*, Ismel Brito 1,2,†, Wilber Romero-Fernandez 1, Michael Di Palma 1,3, Julia Oflijan 4, Kamila Skieterska 5, Jolien Duchou 5, Kathleen Van Craenenbroeck 5, Diana Suárez-Boomgaard 6, Alicia Rivera 6, Diego Guidolin 7, Luigi F. Agnati 1 and Kjell Fuxe 1,* 1 Department of Neuroscience, Karolinska Institutet, Retzius väg 8, 17177 Stockholm, Sweden; E-Mails: [email protected] (I.B.); [email protected] (W.R.-F.); [email protected] (M.D.P.); [email protected] (L.F.A.) 2 IIIA-CSIC, Artificial Intelligence Research Institute, Spanish National Research Council, 08193 Barcelona, Spain 3 Department of Earth, Life and Environmental Sciences, Section of Physiology, Campus Scientifico Enrico Mattei, Urbino 61029, Italy 4 Department of Physiology, Faculty of Medicine, University of Tartu, Tartu 50411, Estonia; E-Mail: [email protected] 5 Laboratory of Eukaryotic Gene Expression and Signal Transduction (LEGEST), Ghent University, 9000 Ghent, Belgium; E-Mails: [email protected] (K.S.); [email protected] (J.D.); [email protected] (K.V.C.) 6 Department of Cell Biology, School of Science, University of Málaga, 29071 Málaga, Spain; E-Mails: [email protected] (D.S.-B.); [email protected] (A.R.) 7 Department of Molecular Medicine, University of Padova, Padova 35121, Italy; E-Mail: [email protected] † These authors contributed equally to this work. -

Purinergic Signaling in the Hallmarks of Cancer
cells Review Purinergic Signaling in the Hallmarks of Cancer Anaí del Rocío Campos-Contreras, Mauricio Díaz-Muñoz and Francisco G. Vázquez-Cuevas * Department of Cellular and Molecular Neurobiology, Instituto de Neurobiología, Universidad Nacional Autónoma de México, Boulevard Juriquilla #3001, Juriquilla Querétaro 76230, Mexico; [email protected] (A.d.R.C.-C.); [email protected] (M.D.-M.) * Correspondence: [email protected]; Tel.: +52-(442)-238-1035 Received: 15 June 2020; Accepted: 2 July 2020; Published: 3 July 2020 Abstract: Cancer is a complex expression of an altered state of cellular differentiation associated with severe clinical repercussions. The effort to characterize this pathological entity to understand its underlying mechanisms and visualize potential therapeutic strategies has been constant. In this context, some cellular (enhanced duplication, immunological evasion), metabolic (aerobic glycolysis, failure in DNA repair mechanisms) and physiological (circadian disruption) parameters have been considered as cancer hallmarks. The list of these hallmarks has been growing in recent years, since it has been demonstrated that various physiological systems misfunction in well-characterized ways upon the onset and establishment of the carcinogenic process. This is the case with the purinergic system, a signaling pathway formed by nucleotides/nucleosides (mainly adenosine triphosphate (ATP), adenosine (ADO) and uridine triphosphate (UTP)) with their corresponding membrane receptors and defined transduction mechanisms. The dynamic equilibrium between ATP and ADO, which is accomplished by the presence and regulation of a set of ectonucleotidases, defines the pro-carcinogenic or anti-cancerous final outline in tumors and cancer cell lines. So far, the purinergic system has been recognized as a potential therapeutic target in cancerous and tumoral ailments. -

Nucleic Acid Ligands Act As a PAM and Agonist Depending on the Intrinsic Ligand Binding State of P2RY2
Nucleic acid ligands act as a PAM and agonist depending on the intrinsic ligand binding state of P2RY2 Masaki Takahashia,1, Ryo Amanoa, Michiru Ozawab, Anna Martinezb, Kazumasa Akitab, and Yoshikazu Nakamuraa,b,1 aProject Division of RNA Medical Science, The Institute of Medical Science, The University of Tokyo, Tokyo 108-8639, Japan; and bRIBOMIC Inc., Tokyo 108-0071, Japan Edited by Brian K. Kobilka, Stanford University School of Medicine, Stanford, CA, and approved March 31, 2021 (received for review September 16, 2020) G protein–coupled receptors (GPCRs) play diverse roles in physiolog- Nonetheless, few aptamers targeting cell-surface proteins, espe- ical processes, and hence the ligands to modulate GPCRs have cially GPCRs, have been reported because of a poor integration of served as important molecules in biological and pharmacological the technologies, despite advances in each relevant technique and approaches. However, the exploration of novel ligands for GPCR expertise. still remains an arduous challenge. In this study, we report a method As aptamers specifically recognize targets with shape comple- for the discovery of nucleic acid ligands against GPCRs by an ad- mentarity, it is vital to choose and prepare selection targets in their vanced RNA aptamer screening technology that employs a virus-like native conformation. Accordingly, although several types of selec- particle (VLP), exposing the GPCR of interest. An array of biochem- tion materials targeting cell-surface proteins (e.g., recombinant ical analyses coupled with a cell-based assay revealed that one of the aptamers raised against purinergic receptor P2Y2 (P2RY2), a proteins, membrane extract, and whole cells) have been used (13, GPCR, exhibits an activation potency to unliganded receptor and 14), the most promising source material appear to be live whole prohibits a further receptor activation by endogenous ligand, be- cells, which ensure the native target structure even in case of un- having like a partial agonist. -

Functional Associations Among G Protein-Coupled Neurotransmitter Receptors in the Human Brain Skirmantas Janušonis
Janušonis BMC Neuroscience 2014, 15:16 http://www.biomedcentral.com/1471-2202/15/16 RESEARCH ARTICLE Open Access Functional associations among G protein-coupled neurotransmitter receptors in the human brain Skirmantas Janušonis Abstract Background: The activity of neurons is controlled by groups of neurotransmitter receptors rather than by individual receptors. Experimental studies have investigated some receptor interactions, but currently little information is available about transcriptional associations among receptors at the whole-brain level. Results: A total of 4950 correlations between 100 G protein-coupled neurotransmitter receptors were examined across 169 brain regions in the human brain using expression data published in the Allen Human Brain Atlas. A large number of highly significant correlations were found, many of which have not been investigated in hypothesis-driven studies. The highest positive and negative correlations of each receptor are reported, which can facilitate the construction of receptor sets likely to be affected by altered transcription of one receptor (such sets always exist, but their members are difficult to predict). A graph analysis isolated two large receptor communities, within each of which receptor mRNA levels were strongly cross-correlated. Conclusions: The presented systematic analysis shows that the mRNA levels of many G protein-coupled receptors are interdependent. This finding is not unexpected, since the brain is a highly integrated complex system. However, the analysis also revealed two novel properties of global brain structure. First, receptor correlations are described by a simple statistical distribution, which suggests that receptor interactions may be guided by qualitatively similar processes. Second, receptors appear to form two large functional communities, which might be differentially affected in brain disorders. -
Supplemental Data
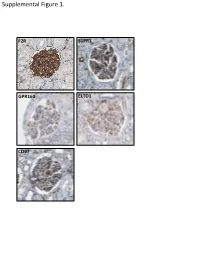
Supplemental Figure 1. F2R S1PR1 GPR160 ELTD1 CD97 Supplemental Figure 2. A B brain heart lung liver kidney spleen testis skm Expression in mouse ssues glom rok Gprc5a Gapdh Human Protein Atlas RNAseq database Supplemental Figure 3. A Gprc5a Pdgfrb Merged CL CL Gprc5a CD31 Merged CL CL B C 1.4 * 1.2 mc 1 matrix 0.8 0.6 0.4 matrix 0.2 0 pod end 1 2 mes3 D Vector Gprc5a E 40 kD - - Gprc5a - acn Supplemental Figure 4. Control 12 month-old KO 12 month-old A B C D E F 1 2 0.8 * 1.5 score 0.6 m µ 1 0.4 0.2 Slits/ 0.5 Mesangial 0 0 Ctrl KO Ctrl KO Supplemental Figure 5. A B 1.2 Vector Gprc5a 1 * 0.8 0.6 * 0.4 * Normalized Density 0.2 0 pEGFR/tEGR pSmad/tSmad TGF-β1 C 1.8 siCON 1.6 siGprc5a * 1.4 * * 1.2 1 0.8 0.6 NormalizedDensity 0.4 0.2 0 pEGFR/tEGR pSmad/tSmad TGF-β1 Supplemental table 1. List of glomerulus-expressed GPCRs as detected by qPCR. Data shown as mean ± standard deviation (Glom=glomerulus, Rok=rest of kidney). GPCR Glom Rok Glom/Rok LPAR6 41892,11 ± 38478,89 1040,06 ± 1370,12 39,28 ELTD1 30275,64 ± 14085,26 33,23 ± 46,99 910,13 GPR116 24020,06 ± 7789,84 55,1 ± 47,75 434,96 PTH1R 15402,81 ± 17644,32 7521,01 ± 3264,57 1,05 CALCRL 14096,09 ± 3854,84 199,06 ± 222,52 69,81 HPRT1 13342,04 ± 10677,69 1824,77 ± 1767,23 6,31 S1PR5 9474,7 ± 10124,3 110,84 ± 29,54 84,48 LPHN2 8645,89 ± 914,74 256,04 ± 293,87 32,77 FZD1 8176,2 ± 4947,45 1321,97 ± 1311,91 5,18 CXCR4 7097,31 ± 4388,91 535,98 ± 640,28 12,24 GPR160 6446,59 ± 1550,07 4816,3 ± 5918,99 0,34 NPY1R 6177,74 ± 6282,76 209,64 ± 296,48 28,47 PTGER4 5323,66 ± 3789,51 179,13 ± 210 28,72 RXFP1 -

Cells and Geneblazer® P2RY2-NFAT-Bla CHO-K1 Cells Conta
Version No.: GeneBLAzer® Validation Packet Page 1 of 5 01Sep08 Optimization of the GeneBLAzer® P2RY2 NFAT-bla CHO-K1 Cell Line GeneBLAzer® P2RY2 CHO-K1 DA Assay Kit GeneBLAzer® P2RY2 NFAT-bla CHO-K1 Cells Catalog Numbers – K1337 and K1722 Cell Line Descriptions GeneBLAzer® P2RY2 CHO-K1 DA (Division Arrested) cells and GeneBLAzer® P2RY2-NFAT-bla CHO-K1 cells contain the human purinergic receptor P2, G protein-coupled, 2 (P2RY2) receptor (Accession # BC012104) stably integrated into the CellSensor® NFAT-bla CHO-K1 cell line. CellSensor® NFAT-bla CHO-K1 (Cat No. K1534) cells contain a beta-lactamase (bla) reporter gene under control of the nuclear factor of activated T cells (NFAT) response element. Division Arrested (DA) cells are available as an Assay Kit, which includes cells and sufficient substrate to analyze 1 x 384-well plate. DA cells are irreversibly division arrested using a low-dose treatment of Mitomycin-C, and have no apparent toxicity or change in cellular signal transduction. Both GeneBLAzer® P2RY2 CHO-K1 DA cells and ® GeneBLAzer P2RY2-NFAT-bla CHO-K1 cells are functionally validated for Z’-factor and EC50 concentrations of Adenosine-5’-triphosphate (ATP); (Figure 1). In addition, GeneBLAzer® P2RY2-NFAT-bla CHO-K1 cells have been tested for assay performance under variable conditions, including DMSO concentration, cell number, stimulation time, and substrate loading time. Additional testing data using alternate stimuli are also included. Target Description The P2Y receptor family is part of a larger receptor family whose physiological effects are mediated by extracellular nucleotide di- and tri-phosphates. The P2 receptor family consists of ion-gated channel receptors (P2X) and G protein coupled receptors (P2Y). -

Microglia and Neuroinflammation: What Place for P2RY12?
International Journal of Molecular Sciences Review Microglia and Neuroinflammation: What Place for P2RY12? Albert Gómez Morillas , Valérie C. Besson † and Dominique Lerouet *,† UMR-S1144–Optimisation Thérapeutique en Neuropsychopharmacologie (OTeN), Faculté de Pharmacie de Paris, 4 Avenue de l’Observatoire, Université de Paris, 75006 Paris, France; [email protected] (A.G.M.); [email protected] (V.C.B.) * Correspondence: [email protected]; Tel.: +33-1-53-73-97-86 † These authors contributed equally to this work. Abstract: Microglia are immune brain cells involved in neuroinflammation. They express a lot of proteins on their surface such as receptors that can be activated by mediators released in the microglial environment. Among these receptors, purinergic receptor expression could be modified depending on the activation status of microglia. In this review, we focus on P2Y receptors and more specifically on P2RY12 that is involved in microglial motility and migration, the first step of neuroinflammation process. We describe the purinergic receptor families, P2RY12 structure, expression and physiological functions. The pharmacological and genetic tools for studying this receptor are detailed thereafter. Last but not least, we report the contribution of microglial P2RY12 to neuroinflammation in acute and chronic brain pathologies in order to better understand P2RY12 microglial role. Keywords: microglia; neuroinflammation; P2RY12; purinergic receptor 1. Introduction Citation: Gómez Morillas, A.; Besson, V.C.; Lerouet, D. Microglia Microglia are the main immune cells in the brain. These plastic cells display a variety and Neuroinflammation: What Place of morphological and functional states in both healthy and pathologic conditions. Numer- for P2RY12? Int. J. -

Treatment of Chronic Neuropathic Pain
Narrative Review Treatment of chronic neuropathic pain: purine receptor modulation Kenneth A. Jacobsona, Luigino Antonio Giancottib, Filomena Laurob, Fatma Muftib, Daniela Salveminib,* 06/11/2020 on 3U6Qur4PG8Cp3fhvloi8qBpY7fujG3dPKasYMD+lWJpib/J9/jG1M3P5/SdPwJzQZopI9kkCkONW3pMktyxp4tovfFowndLubZk7zJkoatOlb7XXAf6/mu3JhfuJ6/VZKl/sopHhTqbQ4RCp2fMF2A== by https://journals.lww.com/pain from Downloaded Downloaded Abstract from https://journals.lww.com/pain Extracellular nucleosides and nucleotides have widespread functions in responding to physiological stress. The “purinome” encompasses 4 G-protein-coupled receptors (GPCRs) for adenosine, 8 GPCRs activated by nucleotides, 7 adenosine 59-triphosphate-gated P2X ion channels, as well as the associated enzymes and transporters that regulate native agonist levels. Purinergic signaling modulators, such as receptor agonists and antagonists, have potential for treating chronic pain. Adenosine and its analogues potently suppress nociception in preclinical models by activating A1 and/or A3 adenosine receptors (ARs), but safely harnessing this pathway to clinically treat pain has not by 3U6Qur4PG8Cp3fhvloi8qBpY7fujG3dPKasYMD+lWJpib/J9/jG1M3P5/SdPwJzQZopI9kkCkONW3pMktyxp4tovfFowndLubZk7zJkoatOlb7XXAf6/mu3JhfuJ6/VZKl/sopHhTqbQ4RCp2fMF2A== been achieved. Both A2AAR agonists and antagonists are efficacious in pain models. Highly selective A3AR agonists offer a novel approach to treat chronic pain. We have explored the structure activity relationship of nucleoside derivatives at this subtype using a computational structure-based approach. Novel A3AR agonists for pain control containing a bicyclic ring system (bicyclo [3.1.0] hexane) in place of ribose were designed and screened using an in vivo phenotypic model, which reflected both pharmacokinetic and pharmacodynamic parameters. High specificity (.10,000-fold selective for A3AR) was achieved with the aid of receptor homology models based on related GPCR structures. These A3AR agonists are well tolerated in vivo and highly efficacious in models of chronic neuropathic pain.