Pollen from Arabidopsis Thaliana and Other Brassicaceae Are Functionally Omniaperturate1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(Cruciferae) – Mustard Family
BRASSICACEAE (CRUCIFERAE) – MUSTARD FAMILY Plant: herbs mostly, annual to perennial, sometimes shrubs; sap sometimes peppery Stem: Root: Leaves: mostly simple but sometimes pinnately divided; alternate, rarely opposite or whorled; no stipules Flowers: mostly perfect, mostly regular (actinomorphic); 4 sepals, 4 petals often forming a cross; 6 stamens with usually 2 outer ones shorter than the inner 4; ovary superior, mostly 2 fused carpels, 1 to many ovules, 1 pistil Fruit: seed pods, often used in classification, many are slender and long (Silique), some broad (Silicle) – see morphology slide Other: a large family, many garden plants such as turnip, radish, and cabbage, also some spices; often termed the Cruciferae family; Dicotyledons Group Genera: 350+ genera; 40+ locally WARNING – family descriptions are only a layman’s guide and should not be used as definitive Flower Morphology in the Brassicaceae (Mustard Family) - flower with 4 sepals, 4 petals (often like a cross, sometimes split or lobed), commonly small, often white or yellow, distinctive fruiting structures often important for ID 2 types of fruiting pods: in addition, fruits may be circular, flattened or angled in cross-section Silicle - (usually <2.5x long as wide), 2-valved with septum (replum) Silique - (usually >2.5x long as wide), 2- valved with septum (replum) Flowers, Many Genera BRASSICACEAE (CRUCIFERAE) – MUSTARD FAMILY Sanddune [Western] Wallflower; Erysimum capitatum (Douglas ex Hook.) Greene var. capitatum Wormseed Wallflower [Mustard]; Erysimum cheiranthoides L. (Introduced) Spreading Wallflower [Treacle Mustard]; Erysimum repandum L. (Introduced) Dame’s Rocket [Dame’s Violet]; Hesperis matronalis L. (Introduced) Purple [Violet] Rocket; Iodanthus pinnatifidus (Michx.) Steud. Michaux's Gladecress; Leavenworthia uniflora (Michx.) Britton [Cow; Field] Cress [Peppergrass]; Lepidium campestre L.) Ait. -

The Vascular Plants of Massachusetts
The Vascular Plants of Massachusetts: The Vascular Plants of Massachusetts: A County Checklist • First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Somers Bruce Sorrie and Paul Connolly, Bryan Cullina, Melissa Dow Revision • First A County Checklist Plants of Massachusetts: Vascular The A County Checklist First Revision Melissa Dow Cullina, Bryan Connolly, Bruce Sorrie and Paul Somers Massachusetts Natural Heritage & Endangered Species Program Massachusetts Division of Fisheries and Wildlife Natural Heritage & Endangered Species Program The Natural Heritage & Endangered Species Program (NHESP), part of the Massachusetts Division of Fisheries and Wildlife, is one of the programs forming the Natural Heritage network. NHESP is responsible for the conservation and protection of hundreds of species that are not hunted, fished, trapped, or commercially harvested in the state. The Program's highest priority is protecting the 176 species of vertebrate and invertebrate animals and 259 species of native plants that are officially listed as Endangered, Threatened or of Special Concern in Massachusetts. Endangered species conservation in Massachusetts depends on you! A major source of funding for the protection of rare and endangered species comes from voluntary donations on state income tax forms. Contributions go to the Natural Heritage & Endangered Species Fund, which provides a portion of the operating budget for the Natural Heritage & Endangered Species Program. NHESP protects rare species through biological inventory, -

Outline of Angiosperm Phylogeny
Outline of angiosperm phylogeny: orders, families, and representative genera with emphasis on Oregon native plants Priscilla Spears December 2013 The following listing gives an introduction to the phylogenetic classification of the flowering plants that has emerged in recent decades, and which is based on nucleic acid sequences as well as morphological and developmental data. This listing emphasizes temperate families of the Northern Hemisphere and is meant as an overview with examples of Oregon native plants. It includes many exotic genera that are grown in Oregon as ornamentals plus other plants of interest worldwide. The genera that are Oregon natives are printed in a blue font. Genera that are exotics are shown in black, however genera in blue may also contain non-native species. Names separated by a slash are alternatives or else the nomenclature is in flux. When several genera have the same common name, the names are separated by commas. The order of the family names is from the linear listing of families in the APG III report. For further information, see the references on the last page. Basal Angiosperms (ANITA grade) Amborellales Amborellaceae, sole family, the earliest branch of flowering plants, a shrub native to New Caledonia – Amborella Nymphaeales Hydatellaceae – aquatics from Australasia, previously classified as a grass Cabombaceae (water shield – Brasenia, fanwort – Cabomba) Nymphaeaceae (water lilies – Nymphaea; pond lilies – Nuphar) Austrobaileyales Schisandraceae (wild sarsaparilla, star vine – Schisandra; Japanese -

Planting Planner
what seeds to sow BRIZA MEDIA Planting ‘GOLDEN BEE’ I love quaking grasses and this selection has a golden tint to the planner seedheads as they age. SLP†. Height (H) 60cm. Sow (S) Best The best seeds to sown in situ in spring. Period of interest (P) sow for 2015 May – September. Winter is the perfect time for studying seed catalogues and planning what to grow. Leading designer Sarah Price gives us a sneak peek of her list WORDS SARAH PRICE PERSICARIA ORIENTALIS Definitely a plant for the back of the border, this tall, multi-branched annual has dramatic, long, deep pink, drooping plumes. Popular in the Victorian era. HA. H 1.8m. S Best sown fresh in autumn. CLOCKWISE FROM TOP: JASON INGRAM X2; STEFFEN HAUSER / GARDEN WORLD IMAGES; NICHOLAS APPLEBY / GARDEN WORLD IMAGES / GARDEN WORLD APPLEBY NICHOLAS IMAGES; / GARDEN WORLD HAUSER X2; STEFFEN INGRAM JASON TOP: FROM CLOCKWISE Can also be sown in early spring. his year my cash-strapped local authority P Late summer to autumn. Twas flooded with unlikely letters of love and appreciation after it set verges and roundabouts alive with a blaze of LATHYRUS annual colour instead of the usual regimented bedding. VERNUS ‘FILIGREE’ It’s proof that broadcasting a packet, or in this case Low-growing, bucketfuls, of mixed seed can have mood-enhancing bushy, hardy effects. It’s not all about live-fast-die-young annuals. perennial sweet pea, Many of my favourite plants are hardy biennials, which invaluable for its take two years to flower, set seed and die, while others are early, purple-blue hardy herbaceous perennials that will live for years. -

Arabidopsis Thaliana
Downloaded from genome.cshlp.org on September 28, 2021 - Published by Cold Spring Harbor Laboratory Press RESEARCH A Physical Map of Chromosome 2 of Arabidopsis thaliana Eve Ann Zachgo, 2,4 Ming Li Wang, 1'2'4 Julia Dewdney, 1'2 David Bouchez, 3 Christine Carnilleri, 3 Stephen Belmonte, 2 Lu Huang, 2 Maureen Dolan, 2 and Howard M. Goodman 1'2'5 1Department of Genetics, Harvard Medical School and 2Department of Molecular Biology, Massachusetts General Hospital, Boston, Massachusetts 02114; 3Laboratoire de Biologie Cellulaire, Institut National de la Recherche Agronomique (INRA), 78026 Versailles CEDEX, France A yeast artificial chromosome (YAC] physical map of chromosome 2 of Arabidopsis thaliana has been constructed by hybridization of 69 DNA markers and 61 YAC end probes to gridded arrays of YAC clones. Thirty-four YACs in four contigs define the chromosome. Complete closure of the map was not attained because some regions of the chromosome were repetitive or were not represented in the YAC library. Based on the sizes of the YACs and their coverage of the chromosome, the length of chromosome 2 is estimated to be at least 18 Mb. These data provide the means for immediately identifying the YACs containing a genetic locus mapped on Arabidopsis chromosome 2. The small flowering plant Arabidopsis thaliana is ters (Maluszynska and Heslop-Harrison 1991; A1- an excellent model system for metabolic, genetic, bini 1994; Copenhaver et al. 1995). We present and developmental studies in plants. Its haploid here a YAC contig physical map of chromosome nuclear genome is small (-100 Mb), consisting of 2 of A. -

Taxa Named in Honor of Ihsan A. Al-Shehbaz
TAXA NAMED IN HONOR OF IHSAN A. AL-SHEHBAZ 1. Tribe Shehbazieae D. A. German, Turczaninowia 17(4): 22. 2014. 2. Shehbazia D. A. German, Turczaninowia 17(4): 20. 2014. 3. Shehbazia tibetica (Maxim.) D. A. German, Turczaninowia 17(4): 20. 2014. 4. Astragalus shehbazii Zarre & Podlech, Feddes Repert. 116: 70. 2005. 5. Bornmuellerantha alshehbaziana Dönmez & Mutlu, Novon 20: 265. 2010. 6. Centaurea shahbazii Ranjbar & Negaresh, Edinb. J. Bot. 71: 1. 2014. 7. Draba alshehbazii Klimeš & D. A. German, Bot. J. Linn. Soc. 158: 750. 2008. 8. Ferula shehbaziana S. A. Ahmad, Harvard Pap. Bot. 18: 99. 2013. 9. Matthiola shehbazii Ranjbar & Karami, Nordic J. Bot. doi: 10.1111/j.1756-1051.2013.00326.x, 10. Plocama alshehbazii F. O. Khass., D. Khamr., U. Khuzh. & Achilova, Stapfia 101: 25. 2014. 11. Alshehbazia Salariato & Zuloaga, Kew Bulletin …….. 2015 12. Alshehbzia hauthalii (Gilg & Muschl.) Salariato & Zuloaga 13. Ihsanalshehbazia Tahir Ali & Thines, Taxon 65: 93. 2016. 14. Ihsanalshehbazia granatensis (Boiss. & Reuter) Tahir Ali & Thines, Taxon 65. 93. 2016. 15. Aubrieta alshehbazii Dönmez, Uǧurlu & M.A.Koch, Phytotaxa 299. 104. 2017. 16. Silene shehbazii S.A.Ahmad, Novon 25: 131. 2017. PUBLICATIONS OF IHSAN A. AL-SHEHBAZ 1973 1. Al-Shehbaz, I. A. 1973. The biosystematics of the genus Thelypodium (Cruciferae). Contrib. Gray Herb. 204: 3-148. 1977 2. Al-Shehbaz, I. A. 1977. Protogyny, Cruciferae. Syst. Bot. 2: 327-333. 3. A. R. Al-Mayah & I. A. Al-Shehbaz. 1977. Chromosome numbers for some Leguminosae from Iraq. Bot. Notiser 130: 437-440. 1978 4. Al-Shehbaz, I. A. 1978. Chromosome number reports, certain Cruciferae from Iraq. -

Plant Life MagillS Encyclopedia of Science
MAGILLS ENCYCLOPEDIA OF SCIENCE PLANT LIFE MAGILLS ENCYCLOPEDIA OF SCIENCE PLANT LIFE Volume 4 Sustainable Forestry–Zygomycetes Indexes Editor Bryan D. Ness, Ph.D. Pacific Union College, Department of Biology Project Editor Christina J. Moose Salem Press, Inc. Pasadena, California Hackensack, New Jersey Editor in Chief: Dawn P. Dawson Managing Editor: Christina J. Moose Photograph Editor: Philip Bader Manuscript Editor: Elizabeth Ferry Slocum Production Editor: Joyce I. Buchea Assistant Editor: Andrea E. Miller Page Design and Graphics: James Hutson Research Supervisor: Jeffry Jensen Layout: William Zimmerman Acquisitions Editor: Mark Rehn Illustrator: Kimberly L. Dawson Kurnizki Copyright © 2003, by Salem Press, Inc. All rights in this book are reserved. No part of this work may be used or reproduced in any manner what- soever or transmitted in any form or by any means, electronic or mechanical, including photocopy,recording, or any information storage and retrieval system, without written permission from the copyright owner except in the case of brief quotations embodied in critical articles and reviews. For information address the publisher, Salem Press, Inc., P.O. Box 50062, Pasadena, California 91115. Some of the updated and revised essays in this work originally appeared in Magill’s Survey of Science: Life Science (1991), Magill’s Survey of Science: Life Science, Supplement (1998), Natural Resources (1998), Encyclopedia of Genetics (1999), Encyclopedia of Environmental Issues (2000), World Geography (2001), and Earth Science (2001). ∞ The paper used in these volumes conforms to the American National Standard for Permanence of Paper for Printed Library Materials, Z39.48-1992 (R1997). Library of Congress Cataloging-in-Publication Data Magill’s encyclopedia of science : plant life / edited by Bryan D. -

Phylogenetic Position and Generic Limits of Arabidopsis (Brassicaceae)
PHYLOGENETIC POSITION Steve L. O'Kane, Jr.2 and Ihsan A. 3 AND GENERIC LIMITS OF Al-Shehbaz ARABIDOPSIS (BRASSICACEAE) BASED ON SEQUENCES OF NUCLEAR RIBOSOMAL DNA1 ABSTRACT The primary goals of this study were to assess the generic limits and monophyly of Arabidopsis and to investigate its relationships to related taxa in the family Brassicaceae. Sequences of the internal transcribed spacer region (ITS-1 and ITS-2) of nuclear ribosomal DNA, including 5.8S rDNA, were used in maximum parsimony analyses to construct phylogenetic trees. An attempt was made to include all species currently or recently included in Arabidopsis, as well as species suggested to be close relatives. Our ®ndings show that Arabidopsis, as traditionally recognized, is polyphyletic. The genus, as recircumscribed based on our results, (1) now includes species previously placed in Cardaminopsis and Hylandra as well as three species of Arabis and (2) excludes species now placed in Crucihimalaya, Beringia, Olimar- abidopsis, Pseudoarabidopsis, and Ianhedgea. Key words: Arabidopsis, Arabis, Beringia, Brassicaceae, Crucihimalaya, ITS phylogeny, Olimarabidopsis, Pseudoar- abidopsis. Arabidopsis thaliana (L.) Heynh. was ®rst rec- netic studies and has played a major role in un- ommended as a model plant for experimental ge- derstanding the various biological processes in netics over a half century ago (Laibach, 1943). In higher plants (see references in Somerville & Mey- recent years, many biologists worldwide have fo- erowitz, 2002). The intraspeci®c phylogeny of A. cused their research on this plant. As indicated by thaliana has been examined by Vander Zwan et al. Patrusky (1991), the widespread acceptance of A. (2000). Despite the acceptance of A. -

Biogeography and Diversification of Brassicales
Molecular Phylogenetics and Evolution 99 (2016) 204–224 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Biogeography and diversification of Brassicales: A 103 million year tale ⇑ Warren M. Cardinal-McTeague a,1, Kenneth J. Sytsma b, Jocelyn C. Hall a, a Department of Biological Sciences, University of Alberta, Edmonton, Alberta T6G 2E9, Canada b Department of Botany, University of Wisconsin, Madison, WI 53706, USA article info abstract Article history: Brassicales is a diverse order perhaps most famous because it houses Brassicaceae and, its premier mem- Received 22 July 2015 ber, Arabidopsis thaliana. This widely distributed and species-rich lineage has been overlooked as a Revised 24 February 2016 promising system to investigate patterns of disjunct distributions and diversification rates. We analyzed Accepted 25 February 2016 plastid and mitochondrial sequence data from five gene regions (>8000 bp) across 151 taxa to: (1) Available online 15 March 2016 produce a chronogram for major lineages in Brassicales, including Brassicaceae and Arabidopsis, based on greater taxon sampling across the order and previously overlooked fossil evidence, (2) examine Keywords: biogeographical ancestral range estimations and disjunct distributions in BioGeoBEARS, and (3) determine Arabidopsis thaliana where shifts in species diversification occur using BAMM. The evolution and radiation of the Brassicales BAMM BEAST began 103 Mya and was linked to a series of inter-continental vicariant, long-distance dispersal, and land BioGeoBEARS bridge migration events. North America appears to be a significant area for early stem lineages in the Brassicaceae order. Shifts to Australia then African are evident at nodes near the core Brassicales, which diverged Cleomaceae 68.5 Mya (HPD = 75.6–62.0). -

Species List For: Labarque Creek CA 750 Species Jefferson County Date Participants Location 4/19/2006 Nels Holmberg Plant Survey
Species List for: LaBarque Creek CA 750 Species Jefferson County Date Participants Location 4/19/2006 Nels Holmberg Plant Survey 5/15/2006 Nels Holmberg Plant Survey 5/16/2006 Nels Holmberg, George Yatskievych, and Rex Plant Survey Hill 5/22/2006 Nels Holmberg and WGNSS Botany Group Plant Survey 5/6/2006 Nels Holmberg Plant Survey Multiple Visits Nels Holmberg, John Atwood and Others LaBarque Creek Watershed - Bryophytes Bryophte List compiled by Nels Holmberg Multiple Visits Nels Holmberg and Many WGNSS and MONPS LaBarque Creek Watershed - Vascular Plants visits from 2005 to 2016 Vascular Plant List compiled by Nels Holmberg Species Name (Synonym) Common Name Family COFC COFW Acalypha monococca (A. gracilescens var. monococca) one-seeded mercury Euphorbiaceae 3 5 Acalypha rhomboidea rhombic copperleaf Euphorbiaceae 1 3 Acalypha virginica Virginia copperleaf Euphorbiaceae 2 3 Acer negundo var. undetermined box elder Sapindaceae 1 0 Acer rubrum var. undetermined red maple Sapindaceae 5 0 Acer saccharinum silver maple Sapindaceae 2 -3 Acer saccharum var. undetermined sugar maple Sapindaceae 5 3 Achillea millefolium yarrow Asteraceae/Anthemideae 1 3 Actaea pachypoda white baneberry Ranunculaceae 8 5 Adiantum pedatum var. pedatum northern maidenhair fern Pteridaceae Fern/Ally 6 1 Agalinis gattingeri (Gerardia) rough-stemmed gerardia Orobanchaceae 7 5 Agalinis tenuifolia (Gerardia, A. tenuifolia var. common gerardia Orobanchaceae 4 -3 macrophylla) Ageratina altissima var. altissima (Eupatorium rugosum) white snakeroot Asteraceae/Eupatorieae 2 3 Agrimonia parviflora swamp agrimony Rosaceae 5 -1 Agrimonia pubescens downy agrimony Rosaceae 4 5 Agrimonia rostellata woodland agrimony Rosaceae 4 3 Agrostis elliottiana awned bent grass Poaceae/Aveneae 3 5 * Agrostis gigantea redtop Poaceae/Aveneae 0 -3 Agrostis perennans upland bent Poaceae/Aveneae 3 1 Allium canadense var. -

Coordination of Biradial-To-Radial Symmetry and Tissue Polarity by HD
ARTICLE https://doi.org/10.1038/s41467-021-24550-6 OPEN Coordination of biradial-to-radial symmetry and tissue polarity by HD-ZIP II proteins ✉ Monica Carabelli1,4, Luana Turchi1, Giorgio Morelli 2, Lars Østergaard 3,5 , Ida Ruberti1,5,6 & ✉ Laila Moubayidin 3,4 Symmetry establishment is a critical process in the development of multicellular organs and requires careful coordination of polarity axes while cells actively divide within tissues. For- 1234567890():,; mation of the apical style in the Arabidopsis gynoecium involves a bilateral-to-radial sym- metry transition, a stepwise process underpinned by the dynamic distribution of the plant morphogen auxin. Here we show that SPATULA (SPT) and the HECATE (HEC) bHLH pro- teins mediate the final step in the style radialisation process and synergistically control the expression of adaxial-identity genes, HOMEOBOX ARABIDOPSIS THALIANA 3 (HAT3) and ARABIDOPSIS THALIANA HOMEOBOX 4 (ATHB4). HAT3/ATHB4 module drives radialisation of the apical style by promoting basal-to-apical auxin flow and via a negative feedback mechanism that finetune auxin distribution through repression of SPT expression and cyto- kinin sensitivity. Thus, this work reveals the molecular basis of axes-coordination and hor- monal cross-talk during the sequential steps of symmetry transition in the Arabidopsis style. 1 Institute of Molecular Biology and Pathology, National Research Council, Rome, Italy. 2 Research Centre for Genomics and Bioinformatics, Council for Agricultural Research and Economics (CREA), Rome, Italy. 3 Department of Crop Genetics, John Innes Centre, Norwich, UK. 4These authors contributed ✉ equally: Monica Carabelli, Laila Moubayidin. 5These authors jointly supervised this work: Lars Østergaard, Ida Ruberti. -
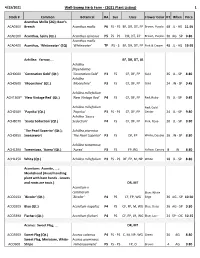
WSHF Catalog
4/23/2021 Well-Sweep Herb Farm - (2021 Plant Listing) 1 Stock # Common Botanical HA Sun Uses Flower ColorHT When Price Acanthus Mollis (2Q); Bear's ACA030X Breech Acanthus mollis P6 FS - PS BF, DR, DT, FP Brown, Purple- 48 JL - AG 11.95 ACA010X Acanthus, Spiny (Qt.) Acanthus spinosus P5 FS - PS DR, DT, FP Brown, Purple- 30 AG - SP 9.80 Acanthus mollis ACA040X Acanthus, `Whitewater' (2Q) `Whitewater' TP PS - S BF, DR, DT, FP Pink & Cream 48 JL - AG 19.95 Achillea: Yarrow, ... BF, DR, DT, LB Achillea filipendulina ACH000X `Coronation Gold' (Qt.) `Coronation Gold' P3 FS CF, DF, FP Gold 36 JL - SP 8.80 Achillea ACH050X `Moonshine' (Qt.) `Moonshine' P3 FS CF, DF, FP Gold 24 JN - SP 9.45 Achillea millefolium ACH130X* `New Vintage Red' (Qt.) `New Vintage Red' P4 FS CF, DF, FP Red, Ruby- 15 JL - SP 9.45 Achillea millefolium Red; Gold ACH250X `Paprika' (Qt.) `Paprika' P3 FS - PS CF, DF, FP Center 24 JL - SP 9.80 Achillea `Saucy ACH807X `Saucy Seduction' (Qt.) Seduction' P4 FS CF, DF, FP Pink, Rose- 20 JL - SP 9.80 `The Pearl Superior' (Qt.); Achillea ptarmica ACH095X Sneezewort `The Pearl Superior' P3 FS DF, FP White; Double 16 JN - SP 8.80 Achillea tomentosa ACH120X Tomentosa, `Aurea' (Qt.) `Aurea' P3 FS FP, RG Yellow, Canary- 8 JN 8.80 ACH125X White (Qt.) Achillea millefolium P3 FS - PS DF, FP, M, NP White 18 JL - SP 8.80 Aconitum: Aconite, ... ; Monkshood (Avoid handling plant with bare hands - Leaves and roots are toxic.) DR, MT Aconitum x cammarum Blue; White ACO015X `Bicolor' (Qt.) `Bicolor' P4 PS CF, FP, WG Edge 36 AG - SP 10.50 ACO020X Blue (Qt.) Aconitum napellus P4 PS CF, FP, M, WG Blue, Deep- 36 AG - SP 9.80 ACO339X Fischeri (Qt.) Aconitum fischeri P4 PS CF, FP, LB, WG Blue, Lav.- 24 SP - OC 10.15 Acorus: Sweet Flag, ..