“Polyphenolic and Proteomic Characterization of Malus Domestica Fruit Affected by Bitter Pit”
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Apples Catalogue 2019
ADAMS PEARMAIN Herefordshire, England 1862 Oct 15 Nov Mar 14 Adams Pearmain is a an old-fashioned late dessert apple, one of the most popular varieties in Victorian England. It has an attractive 'pearmain' shape. This is a fairly dry apple - which is perhaps not regarded as a desirable attribute today. In spite of this it is actually a very enjoyable apple, with a rich aromatic flavour which in apple terms is usually described as Although it had 'shelf appeal' for the Victorian housewife, its autumnal colouring is probably too subdued to compete with the bright young things of the modern supermarket shelves. Perhaps this is part of its appeal; it recalls a bygone era where subtlety of flavour was appreciated - a lovely apple to savour in front of an open fire on a cold winter's day. Tree hardy. Does will in all soils, even clay. AERLIE RED FLESH (Hidden Rose, Mountain Rose) California 1930’s 19 20 20 Cook Oct 20 15 An amazing red fleshed apple, discovered in Aerlie, Oregon, which may be the best of all red fleshed varieties and indeed would be an outstandingly delicious apple no matter what color the flesh is. A choice seedling, Aerlie Red Flesh has a beautiful yellow skin with pale whitish dots, but it is inside that it excels. Deep rose red flesh, juicy, crisp, hard, sugary and richly flavored, ripening late (October) and keeping throughout the winter. The late Conrad Gemmer, an astute observer of apples with 500 varieties in his collection, rated Hidden Rose an outstanding variety of top quality. -

Foundation Arboretum Wespelaar Year Report 2019
Foundation Arboretum Wespelaar Year Report 2019 2019 started with an early spring: by mid-March the early magnolias were in full flower. Temperatures kept rising and the heat record was broken in July, making the summer of 2019 one to be recorded in history books. Luckily, small rain showers and increased irrigation capacities ensured that we lost very few plants. In March 2019, the construction of the Artois Pavilion started in the Artois Meadow, at the end of two important vistas. The design is inspired by the old pavilion along the canal in the Park of Wespelaar. In the new Arboretum de Marche, a total of 189 trees have been planted in the past two years. In collaboration with Natagora, five ponds were created and a 5ha plot was sown with wild seed to create a meadow of native grasses and flowers. The building permit for the visitors centre and technical facilities was obtained. 2019 was a very active year for both Arboretum Wespelaar and Arboretum de Marche, as is described in more detail in this Year Report. Arboretum Wespelaar – Year Report 2019 1 THE COLLECTIONS ‘Patty’ can be planted in the Arboretum in due time. The living collection of woody plants in the Arboretum currently (as of 30 January 2019) Once again, our winter was not worth contains 5,116 specimens representing 2,340 mentioning and by mid-March we could have different taxa (versus 16,376 specimens and opened the Arboretum for our visitors because 4,955 taxa on the whole of the estate). These the early magnolias were already in full flower! numbers include the 629 new accessions on On March 11th however, we had a serious the estate during 2019 of which 108 (or 17%) storm with accompanying damage and much are of documented wild origin. -

2019 Newsletter
Front page: Allen’s greeting, something new 2019 NEWSLETTER A Message From Our President & Owner, EVENT CALENDAR Cooler mornings and valley fog below the orchard remind us all that it’s about apple time! Nature has blessed us with August 19th a beautiful crop of apples with exceptionally good fruit size. Opening Day Compared to recent years, some varieties may be picked a little later this year so be sure to give us a call or check our website to September 27th - 29th make sure your favorite apple is available. I enjoy every apple Gays Mills Apple Festival variety we grow, but Evercrisp has me as excited as Honeycrisp. October 5th - 6th Harvested in late October and stored in a refrigerator, Evercrisp Sunrise Samples Weekend is a fantastic eating experience in the winter months. Our family has been growing apples since 1934 and we have never tasted October 12th - 13th another winter apple like Evercrisp! Family Fun Weekend I hope you all enjoyed our newly expanded sales area and October 19th - 20th bathrooms added in 2018. This year we have made additional Harvest Celebration exciting improvements with a new gift area, live apple packing & Helicopter Rides TV, and a working model train for young and old to enjoy. Our famous cider donuts will be back- made fresh every day. Please (weather permitting ) enjoy our free apple and cider samples along with many of the October 21st - December 16th other products we sell. Gift Box Shipping Begins Don’t forget our online store. We feature many of the October 26th - 27th items available here and have made it far easier to order gift pack Trick or Treat Weekend apples this year from home. -

Osher Lifelong Learning Institute
USDA-ARS National Plant Germplasm System Conservation of Fruit & Nut Genetic Resources Joseph Postman Plant Pathologist & Curator National Clonal Germplasm Repository Corvallis, Oregon May 2010 Mission: Collect – Preserve Evaluate – Enhance - Distribute World Diversity of Plant Genetic Resources for Improving the Quality and Production of Economic Crops Important to U.S. and World Agriculture Apple Accessions at Geneva Malus angustifolia ( 59 Accessions) Malus sikkimensis ( 14 Accessions) Malus baccata ( 67 Accessions) Malus sp. ( 41 Accessions) Malus bhutanica ( 117 Accessions) Malus spectabilis ( 9 Accessions) Malus brevipes ( 2 Accessions) Malus sylvestris ( 70 Accessions) Malus coronaria ( 98 Accessions) Malus toringo ( 122 Accessions) Malus domestica ( 1,389 Accessions) Malus transitoria ( 63 Accessions) Malus doumeri ( 2 Accessions) Malus trilobata ( 2 Accessions) Malus florentina ( 4 Accessions) Malus tschonoskii ( 3 Accessions) Malus floribunda ( 12 Accessions) Malus x adstringens ( 2 Accessions) Malus fusca ( 147 Accessions) Malus x arnoldiana ( 2 Accessions) Malus halliana ( 15 Accessions) Malus x asiatica ( 20 Accessions) Malus honanensis ( 4 Accessions) Malus x astracanica ( 1 Accessions) Malus hupehensis ( 185 Accessions) Malus x atrosanguinea ( 2 Accessions) Malus hybrid ( 337 Accessions) Malus x dawsoniana ( 2 Accessions) Malus ioensis ( 72 Accessions) Malus x hartwigii ( 5 Accessions) Malus kansuensis ( 45 Accessions) Malus x magdeburgensis ( 2 Accessions) Malus komarovii ( 1 Accessions) Malus x micromalus ( 25 Accessions) -

CHLOROPLAST Matk GENE PHYLOGENY of SOME IMPORTANT SPECIES of PLANTS
AKDENİZ ÜNİVERSİTESİ ZİRAAT FAKÜLTESİ DERGİSİ, 2005, 18(2), 157-162 CHLOROPLAST matK GENE PHYLOGENY OF SOME IMPORTANT SPECIES OF PLANTS Ayşe Gül İNCE1 Mehmet KARACA2 A. Naci ONUS1 Mehmet BİLGEN2 1Akdeniz University Faculty of Agriculture Department of Horticulture, 07059 Antalya, Turkey 2Akdeniz University Faculty of Agriculture Department of Field Crops, 07059 Antalya, Turkey Correspondence addressed E-mail: [email protected] Abstract In this study using the chloroplast matK DNA sequence, a chloroplast-encoded locus that has been shown to be much more variable than many other genes, from one hundred and forty two plant species belong to the families of 26 plants we conducted a study to contribute to the understanding of major evolutionary relationships among the studied plant orders, families genus and species (clades) and discussed the utilization of matK for molecular phylogeny. Determined genetic relationship between the species or genera is very valuable for genetic improvement studies. The chloroplast matK gene sequences ranging from 730 to 1545 nucleotides were downloaded from the GenBank database. These DNA sequences were aligned using Clustal W program. We employed the maximum parsimony method for phylogenetic reconstruction using PAUP* program. Trees resulting from the parsimony analyses were similar to those generated earlier using single or multiple gene analyses, but our analyses resulted in strict consensus tree providing much better resolution of relationships among major clades. We found that gymnosperms (Pinus thunbergii, Pinus attenuata and Ginko biloba) were different from the monocotyledons and dicotyledons. We showed that Cynodon dactylon, Panicum capilare, Zea mays and Saccharum officiarum (all are in the C4 metabolism) were improved from a common ancestors while the other cereals Triticum Avena, Hordeum, Oryza and Phalaris were evolved from another or similar ancestors. -
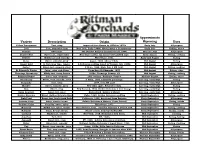
Variety Description Origin Approximate Ripening Uses
Approximate Variety Description Origin Ripening Uses Yellow Transparent Tart, crisp Imported from Russia by USDA in 1870s Early July All-purpose Lodi Tart, somewhat firm New York, Early 1900s. Montgomery x Transparent. Early July Baking, sauce Pristine Sweet-tart PRI (Purdue Rutgers Illinois) release, 1994. Mid-late July All-purpose Dandee Red Sweet-tart, semi-tender New Ohio variety. An improved PaulaRed type. Early August Eating, cooking Redfree Mildly tart and crunchy PRI release, 1981. Early-mid August Eating Sansa Sweet, crunchy, juicy Japan, 1988. Akane x Gala. Mid August Eating Ginger Gold G. Delicious type, tangier G Delicious seedling found in Virginia, late 1960s. Mid August All-purpose Zestar! Sweet-tart, crunchy, juicy U Minn, 1999. State Fair x MN 1691. Mid August Eating, cooking St Edmund's Pippin Juicy, crisp, rich flavor From Bury St Edmunds, 1870. Mid August Eating, cider Chenango Strawberry Mildly tart, berry flavors 1850s, Chenango County, NY Mid August Eating, cooking Summer Rambo Juicy, tart, aromatic 16th century, Rambure, France. Mid-late August Eating, sauce Honeycrisp Sweet, very crunchy, juicy U Minn, 1991. Unknown parentage. Late Aug.-early Sept. Eating Burgundy Tart, crisp 1974, from NY state Late Aug.-early Sept. All-purpose Blondee Sweet, crunchy, juicy New Ohio apple. Related to Gala. Late Aug.-early Sept. Eating Gala Sweet, crisp New Zealand, 1934. Golden Delicious x Cox Orange. Late Aug.-early Sept. Eating Swiss Gourmet Sweet-tart, juicy Switzerland. Golden x Idared. Late Aug.-early Sept. All-purpose Golden Supreme Sweet, Golden Delcious type Idaho, 1960. Golden Delicious seedling Early September Eating, cooking Pink Pearl Sweet-tart, bright pink flesh California, 1944, developed from Surprise Early September All-purpose Autumn Crisp Juicy, slow to brown Golden Delicious x Monroe. -

Northstar Fire Department, Fire Resistant Landscaping Plant List
Northstar Fire Department, Fire Resistant Landscaping Plant List Many common plants naturally resist fire and can even slow its spread. In general, irrigated and well maintained leafy plants burn slowly. By replacing highly flammable vegetation with these fire resistant recommended species, you can significantly improve the statistical survivability of your home when a wildfire threatens. Courtesy of the “Villager Nursery” in Truckee, this list of fire resistive plants should be considered when enhancing or adding landscape. Here are some other key components to remember when selecting appropriate fire resistant landscaping plants. Choose plants that don’t produce much litter such as dead branches and twigs Plants that produce oils, resins or waxes should be avoided When planting from the approved list, appropriately space plants to prevent fire spread Never plant under conifer trees, this can create a ladder effect which allows smaller ground fires the ability to transfer into larger trees creating crown fires To help ensure their fire resistance, plants must be maintained, watered, and pruned. Routine care will provide you with an attractive defensible space landscape and help ensure the survival of your property in the event of a wildfire. Northstar Fire Department enforces both State and District fire codes that may directly affect your landscape. Northstar Fire Department recommends that prior to changing your landscape you consult with us in order to make sure your property will be compliant with applicable fire codes. Trees Common Name Scientific Name Please note that there Apple Malus spp. may be other vegetative Bigtooth Maple Acer grandidentatum species that are Cherry Prunus spp. -

State of New York City's Plants 2018
STATE OF NEW YORK CITY’S PLANTS 2018 Daniel Atha & Brian Boom © 2018 The New York Botanical Garden All rights reserved ISBN 978-0-89327-955-4 Center for Conservation Strategy The New York Botanical Garden 2900 Southern Boulevard Bronx, NY 10458 All photos NYBG staff Citation: Atha, D. and B. Boom. 2018. State of New York City’s Plants 2018. Center for Conservation Strategy. The New York Botanical Garden, Bronx, NY. 132 pp. STATE OF NEW YORK CITY’S PLANTS 2018 4 EXECUTIVE SUMMARY 6 INTRODUCTION 10 DOCUMENTING THE CITY’S PLANTS 10 The Flora of New York City 11 Rare Species 14 Focus on Specific Area 16 Botanical Spectacle: Summer Snow 18 CITIZEN SCIENCE 20 THREATS TO THE CITY’S PLANTS 24 NEW YORK STATE PROHIBITED AND REGULATED INVASIVE SPECIES FOUND IN NEW YORK CITY 26 LOOKING AHEAD 27 CONTRIBUTORS AND ACKNOWLEGMENTS 30 LITERATURE CITED 31 APPENDIX Checklist of the Spontaneous Vascular Plants of New York City 32 Ferns and Fern Allies 35 Gymnosperms 36 Nymphaeales and Magnoliids 37 Monocots 67 Dicots 3 EXECUTIVE SUMMARY This report, State of New York City’s Plants 2018, is the first rankings of rare, threatened, endangered, and extinct species of what is envisioned by the Center for Conservation Strategy known from New York City, and based on this compilation of The New York Botanical Garden as annual updates thirteen percent of the City’s flora is imperiled or extinct in New summarizing the status of the spontaneous plant species of the York City. five boroughs of New York City. This year’s report deals with the City’s vascular plants (ferns and fern allies, gymnosperms, We have begun the process of assessing conservation status and flowering plants), but in the future it is planned to phase in at the local level for all species. -

A Small Molecule but Important for Salt Stress Tolerance in Plants
Review Melatonin: A Small Molecule but Important for Salt Stress Tolerance in Plants Haoshuang Zhan 1,†, Xiaojun Nie 1,†, Ting Zhang 1, Shuang Li 1, Xiaoyu Wang 1, Xianghong Du 1, Wei Tong 1,* and Weining Song 1,2,* 1 State Key Laboratory of Crop Stress Biology in Arid Areas, College of Agronomy and Yangling Branch of China Wheat Improvement Center, Northwest A&F University, Yangling 712100, China; [email protected] (H.Z.); [email protected] (X.N.); [email protected] (T.Z.); [email protected] (S.L.); [email protected] (W.X.); [email protected] (X.D.) 2 ICARDA-NWSUAF Joint Research Center for Agriculture Research in Arid Areas, Yangling 712100, China * Corresponding authors: [email protected] (W.T.); [email protected] (W.S.); Tel.: +86-29-8708-2984 (W.S.); Fax: +86-29-8708-2203 (W.S.) † These authors contributed equally to this work. Received: 1 January 2019; Accepted: 4 February 2019; Published: 7 February 2019 Abstract: Salt stress is one of the most serious limiting factors in worldwide agricultural production, resulting in huge annual yield loss. Since 1995, melatonin (N-acetyl-5-methoxytryptamine)—an ancient multi-functional molecule in eukaryotes and prokaryotes—has been extensively validated as a regulator of plant growth and development, as well as various stress responses, especially its crucial role in plant salt tolerance. Salt stress and exogenous melatonin lead to an increase in endogenous melatonin levels, partly via the phyto-melatonin receptor CAND2/PMTR1. Melatonin plays important roles, as a free radical scavenger and antioxidant, in the improvement of antioxidant systems under salt stress. -

Malussylvestris Family: Rosaceae Apple
Malus sylvestris Family: Rosaceae Apple Apple (Malus spp.) consists of 30+ species that occur on both sides of the Atlantic in northern temperate zones. Its wood can be confused with pear (Pyrus spp.) and other “fruitwoods” in the rose family (Rosaceae). Malus is the classical Latin name for apple. Apple hybridizes with North American crab apples. Malus angustifolia-American crab apple, buncombe crab apple, crab apple, crabtree, narrowleaf crab, narrowleaf crab apple, southern crab, southern crab apple, wild crab, wild crab apple Malus coronaria-Alabama crab, Allegheny crab, American crab, American crab apple, Biltmore crab apple, Buncombe crab, crab, crab apple, Dawson crab, Dunbar crab, fragrant crab, garland tree, lanceleaf crab apple, Missouri crab, sweet crab apple, sweet-scented crab, sweet wild crab, wild crab, wild sweet crab Malus fusca-crab apple, Oregon crab, Oregon crab apple, Pacific crab apple, western crab apple, wild crab apple Malus ioensis-Bechel crab, crab apple, Iowa crab, Iowa crab apple, prairie crab, prairie crab apple, wild crab, wild crab apple Malus sylvestris-apple, common apple, wild apple. Distribution Apple is a cultivated fruit tree, persistent, escaped and naturalized locally across southern Canada, in eastern continental United States, and from Washington south to California. Native to Europe and west Asia. Apple grows wild in the southern part of Great Britain and Scandinavia and is found throughout Europe and southwestern Asia. It is planted in most temperate climates The Tree The tree rarely reaches 30 ft (9 m), with a small crooked bole to 1 ft (0.3 m) in diameter. The Wood General Apple wood has a reddish gray heartwood and light reddish sapwood (12 to 30 rings of sapwood). -

CHARACTERISTICS of BLOOMING, FLORAL NECTARIES and NECTAR of Malus Sargentii Rehd
ACTA AGROBOTANICA Vol. 62 (1): 17–25 2009 CHARACTERISTICS OF BLOOMING, FLORAL NECTARIES AND NECTAR OF Malus sargentii Rehd. Elżbieta Weryszko-Chmielewska, Marta Dmitruk Department of Botany, University of Life Sciences in Lublin, Akademicka 15, 20-950 Lublin, Poland e-mail: [email protected] Received: 15.02.2009 Abstract flowers, alongside coloured buds flowers with whi- In the years 2007-2008, the flowering biology of Ma- te petals occur on the tree. Red, shining fruits with lus sargentii, an ornamental apple tree native to Japan, was a diameter of up to 1 cm, growing on long, thin stalks, studied in the conditions of Lublin (Poland). The daily rate also contribute to the ornamental value of this tree of flower opening, flowering duration and flower visitation (Bugał a , 2000). by insects were determined. The amount of nectar produced Malus sargentii fruits are used for ornamental per flower and sugar content in the nectar were investigated. plant arrangements and for decorating confectionary The size of nectaries and the micromorphology of their surface products. This species is utilized as one of pollinators were examined using light and scanning electron microscopy. in commercial orchards. It was found that the greatest amount of flowers opened be- tween 11.00 and 13.00. During this time, the largest number Different apple species are recognised as an of insects was observed in the flowers. Bees (90%) were pre- important source of nectar (J a b ł o ń ski, 1986; G u - dominant among the insects, with a much smaller number of lyás et al. -
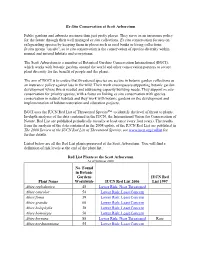
IUCN Red List of Threatened Species™ to Identify the Level of Threat to Plants
Ex-Situ Conservation at Scott Arboretum Public gardens and arboreta are more than just pretty places. They serve as an insurance policy for the future through their well managed ex situ collections. Ex situ conservation focuses on safeguarding species by keeping them in places such as seed banks or living collections. In situ means "on site", so in situ conservation is the conservation of species diversity within normal and natural habitats and ecosystems. The Scott Arboretum is a member of Botanical Gardens Conservation International (BGCI), which works with botanic gardens around the world and other conservation partners to secure plant diversity for the benefit of people and the planet. The aim of BGCI is to ensure that threatened species are secure in botanic garden collections as an insurance policy against loss in the wild. Their work encompasses supporting botanic garden development where this is needed and addressing capacity building needs. They support ex situ conservation for priority species, with a focus on linking ex situ conservation with species conservation in natural habitats and they work with botanic gardens on the development and implementation of habitat restoration and education projects. BGCI uses the IUCN Red List of Threatened Species™ to identify the level of threat to plants. In-depth analyses of the data contained in the IUCN, the International Union for Conservation of Nature, Red List are published periodically (usually at least once every four years). The results from the analysis of the data contained in the 2008 update of the IUCN Red List are published in The 2008 Review of the IUCN Red List of Threatened Species; see www.iucn.org/redlist for further details.