Selection of Effective Cocrystals Former for Dissolution Rate Improvement Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dorset Medicines Advisory Group
DORSET CARDIOLOGY WORKING GROUP GUIDELINE FOR CALCIUM CHANNEL BLOCKERS IN HYPERTENSION SUMMARY The pan-Dorset cardiology working group continues to recommend the use of amlodipine (a third generation dihydropyridine calcium-channel blocker) as first choice calcium channel blocker on the pan-Dorset formulary for hypertension. Lercanidipine is second choice, lacidipine third choice and felodipine is fourth choice. This is due to preferable side effect profiles in terms of ankle oedema and relative costs of the preparations. Note: where angina is the primary indication or is a co-morbidity prescribers must check against the specific product characteristics (SPC) for an individual drug to confirm this is a licensed indication. N.B. Lacidipine and lercandipine are only licensed for use in hypertension. Chapter 02.06.02 CCBs section of the Formulary has undergone an evidence-based review. A comprehensive literature search was carried out on NHS Evidence, Medline, EMBASE, Cochrane Database, and UK Duets. This was for recent reviews or meta-analyses on calcium channel blockers from 2009 onwards (comparative efficacy and side effects) and randomised controlled trials (RCTs). REVIEW BACKGROUND Very little good quality evidence exists. No reviews, meta-analyses or RCTs were found covering all calcium channel blockers currently on the formulary. Another limitation was difficulty obtaining full text original papers for some of the references therefore having to use those from more obscure journals instead. Some discrepancies exist between classification of generations of dihydropyridine CCBs, depending upon the year of publication of the reference/authors’ interpretation. Dihydropyridine (DHP) CCBs tend to be more potent vasodilators than non-dihydropyridine (non-DHP) CCBs (diltiazem, verapamil), but the latter have greater inotropic effects. -

PMBJP Product.Pdf
Sr. Drug Generic Name of the Medicine Unit Size MRP Therapeutic Category No. Code Analgesic & Antipyretic / Muscle 1 1 Aceclofenac 100mg and Paracetamol 325 mg Tablet 10's 10's 8.00 relaxants Analgesic & Antipyretic / Muscle 2 2 Aceclofenac Tablets IP 100mg 10's 10's 4.37 relaxants Acetaminophen 325 + Tramadol Hydrochloride 37.5 film Analgesic & Antipyretic / Muscle 3 4 10's 8.00 coated Tablet 10's relaxants Analgesic & Antipyretic / Muscle 4 5 ASPIRIN Tablets IP 150 mg 14's 14's 2.70 relaxants DICLOFENAC 50 mg+ PARACETAMOL 325 mg+ Analgesic & Antipyretic / Muscle 5 6 10's 11.30 CHLORZOXAZONE 500 mg Tablets 10's relaxants Diclofenac Sodium 50mg + Serratiopeptidase 10mg Tablet Analgesic & Antipyretic / Muscle 6 8 10's 12.00 10's relaxants Analgesic & Antipyretic / Muscle 7 9 Diclofenac Sodium (SR) 100 mg Tablet 10's 10's 6.12 relaxants Analgesic & Antipyretic / Muscle 8 10 Diclofenac Sodium 25mg per ml Inj. IP 3 ml 3 ml 2.00 relaxants Analgesic & Antipyretic / Muscle 9 11 Diclofenac Sodium 50 mg Tablet 10's 10's 2.90 relaxants Analgesic & Antipyretic / Muscle 10 12 Etoricoxilb Tablets IP 120mg 10's 10's 33.00 relaxants Analgesic & Antipyretic / Muscle 11 13 Etoricoxilb Tablets IP 90mg 10's 10's 25.00 relaxants Analgesic & Antipyretic / Muscle 12 14 Ibuprofen 400 mg + Paracetamol 325 mg Tablet 10's 15's 5.50 relaxants Analgesic & Antipyretic / Muscle 13 15 Ibuprofen 200 mg film coated Tablet 10's 10's 1.80 relaxants Analgesic & Antipyretic / Muscle 14 16 Ibuprofen 400 mg film coated Tablet 10's 15's 3.50 relaxants Analgesic & Antipyretic -
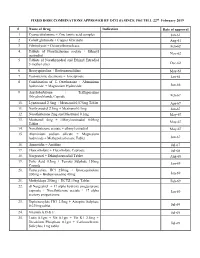
FIXED DOSE COMBINATIONS APPROVED by DCG (I) SINCE 1961 TILL 22Nd February 2019
FIXED DOSE COMBINATIONS APPROVED BY DCG (I) SINCE 1961 TILL 22nd February 2019 # Name of Drug Indication Date of approval 1. Cyanocobalamine + Zinc tannic acid complex Jan-61 2. Cobalt glutamate + Copper Glycinate Aug-61 3. Fibrinolysin + Desoxyribonuclease Feb-62 4. Tablets of Norethisterone acetate + Ethinyl Nov-62 oestradiol 5. Tablets of Norethynodrel and Ethinyl Estradiol 3-methyl ether Dec-62 6. Broxyquinoline + Brobenzoxalidine May-63 7. Testosterone decanoate + Isocaproate Jan-64 8. Combination of L Oxethazaine + Aluminium hydroxide + Magnesium Hydroxide Jun-66 9. Amylobarbitone + Trifluperazine Dihydrochloride Capsule Feb-67 10. Lynestronol 2.5mg + Mestranol 0.075mg Tablet Apr-67 11. Northynodrel 2.5mg + Mestranol 0.1mg Jun-67 12. Norethisterone 2mg and Mestranol 0.1mg May-67 13. Mestranol 4mg + Ethinyloestradial 0.05mg May-67 Tablet 14. Norethisterane acetate + ethinyl estradiol May-67 15. Aluminium sodium silicate + Magnesium hydroxide + Methypolysiloxane Tablet Jun-67 16. Ammoidin + Amidine Jul-67 17. Fluocortolene + Flucortolene Caproate Jul-68 18. Norgestrel + Ethinyloestradiol Tablet Aug-68 19. Folic Acid 0.5mg + Ferrous Sulphate 150mg Jan-69 Capsule 20. Tetracycline HCl 250mg + Broxyquinoline 200mg + Brobenzoxadine 40mg Jan-69 21. Methyldopa 250mg + HCTZ 15mg Tablet Feb-69 22. dl Norgestrel + 17 alpha hydroxy progesterone caproate + Norethisterone acetate + 17 alpha Jan-69 acetoxy progesterone 23. Diphenoxylate HCl 2.5mg + Atropine Sulphate 0.025mg tablet Jul-69 24. Vitamin A,D & E Jul-69 25. Lutin 0.1gm + Vit 0.1gm + Vit K1 2.5mg + Dicalcium Phosphate 0.1gm + Carlozochrome Jul-69 Salicylate 1mg tablet 26. Vit K 1 5mg + Calcium Lactolionate 100 m g+ Carlozocrome Salicylate 2.5mg + Phenol 0.5% Jul-69 + Lignocaine Hcl 1% injection 27. -

Drug Name Plate Number Well Location % Inhibition, Screen Axitinib 1 1 20 Gefitinib (ZD1839) 1 2 70 Sorafenib Tosylate 1 3 21 Cr
Drug Name Plate Number Well Location % Inhibition, Screen Axitinib 1 1 20 Gefitinib (ZD1839) 1 2 70 Sorafenib Tosylate 1 3 21 Crizotinib (PF-02341066) 1 4 55 Docetaxel 1 5 98 Anastrozole 1 6 25 Cladribine 1 7 23 Methotrexate 1 8 -187 Letrozole 1 9 65 Entecavir Hydrate 1 10 48 Roxadustat (FG-4592) 1 11 19 Imatinib Mesylate (STI571) 1 12 0 Sunitinib Malate 1 13 34 Vismodegib (GDC-0449) 1 14 64 Paclitaxel 1 15 89 Aprepitant 1 16 94 Decitabine 1 17 -79 Bendamustine HCl 1 18 19 Temozolomide 1 19 -111 Nepafenac 1 20 24 Nintedanib (BIBF 1120) 1 21 -43 Lapatinib (GW-572016) Ditosylate 1 22 88 Temsirolimus (CCI-779, NSC 683864) 1 23 96 Belinostat (PXD101) 1 24 46 Capecitabine 1 25 19 Bicalutamide 1 26 83 Dutasteride 1 27 68 Epirubicin HCl 1 28 -59 Tamoxifen 1 29 30 Rufinamide 1 30 96 Afatinib (BIBW2992) 1 31 -54 Lenalidomide (CC-5013) 1 32 19 Vorinostat (SAHA, MK0683) 1 33 38 Rucaparib (AG-014699,PF-01367338) phosphate1 34 14 Lenvatinib (E7080) 1 35 80 Fulvestrant 1 36 76 Melatonin 1 37 15 Etoposide 1 38 -69 Vincristine sulfate 1 39 61 Posaconazole 1 40 97 Bortezomib (PS-341) 1 41 71 Panobinostat (LBH589) 1 42 41 Entinostat (MS-275) 1 43 26 Cabozantinib (XL184, BMS-907351) 1 44 79 Valproic acid sodium salt (Sodium valproate) 1 45 7 Raltitrexed 1 46 39 Bisoprolol fumarate 1 47 -23 Raloxifene HCl 1 48 97 Agomelatine 1 49 35 Prasugrel 1 50 -24 Bosutinib (SKI-606) 1 51 85 Nilotinib (AMN-107) 1 52 99 Enzastaurin (LY317615) 1 53 -12 Everolimus (RAD001) 1 54 94 Regorafenib (BAY 73-4506) 1 55 24 Thalidomide 1 56 40 Tivozanib (AV-951) 1 57 86 Fludarabine -

In Vivo Effect of PC-SPES on Prostate Growth and Hepatic CYP3A Expression in Rats
JPET Fast Forward. Published on April 3, 2003 as DOI: 10.1124/jpet.102.048645 JPETThis Fast article Forward. has not been Published copyedited and on formatted. April 3, The 2003 final asversion DOI:10.1124/jpet.102.048645 may differ from this version. JPET #48645 In Vivo Effect of PC-SPES on Prostate Growth and Hepatic CYP3A Expression in Rats Teri Wadsworth, Hataya Poonyagariyagorn, Elinore Sullivan, Dennis Koop and Charles E. Roselli. Downloaded from Department of Physiology and Pharmacology Oregon Health & Science University, Portland, OR. jpet.aspetjournals.org at ASPET Journals on September 26, 2021 1 Copyright 2003 by the American Society for Pharmacology and Experimental Therapeutics. JPET Fast Forward. Published on April 3, 2003 as DOI: 10.1124/jpet.102.048645 This article has not been copyedited and formatted. The final version may differ from this version. JPET #48645 Running Title: In vivo effects of PC-SPES Correspondence: Dr. Charles E. Roselli, Department of Physiology and Pharmacology L334, Oregon Health Sciences University, 3181 SW Sam Jackson Park Road, Portland, OR 97201-3098, Tel (503) 494-5837, FAX (503) 494-4352, email: [email protected] Number of text pages: 20 number of tables: 4 Downloaded from number of figures: 4 number of references: 40 jpet.aspetjournals.org number of words in the Abstract: 250 number of words the Introduction: 748 number of words in the Discussion: 1478 at ASPET Journals on September 26, 2021 nonstandard abbreviations: National Institute of Diabetes and Digestive and Kidney Disease (NIDDK); -

Mechanochemical Synthesis of a Cocrystal of Two Supramolecular
crystals Article Mechanochemical Synthesis of a Cocrystal of Two Supramolecular Hydrogen-Bonded Aggregates of 1,3,6,8-Tetraazatricyclo[4.3.1.13,8]undecane (TATU) with 4-tert-Butylphenol Bearing Different Hydrogen Bonding Interactions Augusto Rivera 1,*, Jicli José Rojas 1, Jaime Ríos-Motta 1 and Michael Bolte 2 ID 1 Departamento de Química, Facultad de Ciencias, Universidad Nacional de Colombia, Sede Bogotá, Carrera 30 No. 45-03, Bogotá,Código Postal 111321, Colombia; [email protected] (J.J.R.); [email protected] (J.R.-M.) 2 Institut für Anorganische Chemie, J. W. Goethe-Universität Frankfurt, Max-von Laue-Str., 7, 60438 Frankfurt/Main, Germany; [email protected] * Correspondence: [email protected]; Tel.: +57-1-316-5000 Received: 2 July 2018; Accepted: 20 July 2018; Published: 30 July 2018 Abstract: The synthesis and single crystal structure of a new cocrystal, which is composed of OHphenolic···OHphenolic···Naminalic supramolecular heterosynthons assembled from 4-tert-butylphenol and the macrocyclic aminal TATU, is presented. This cocrystal was prepared by solvent-free assisted grinding, which is a commonly used mechanochemical method. Crystal structure, supramolecular assembly through hydrogen bonding interactions as well as the physical and spectroscopic properties of the title cocrystal are presented in this paper. Keywords: supramolecular chemistry; crystal engineering; cocrystals; hydrogen bonds; noncovalent interactions; aminal structure 1. Introduction Noncovalent interactions, such as electrostatic forces, H-bonds, halogen bonds, CH–π, π–π stacking, cation–π, anion–π, lone pair–π associations and other weak forces, play significant roles in a variety of fields, including molecular recognition, host–guest chemistry, crystal engineering, supramolecular chemistry, biochemistry, pharmaceutical chemistry, and materials science [1,2]. -

SNI May-Jun-2011 Cover Final
OPEN ACCESS Editor-in-Chief: Surgical Neurology International James I. Ausman, MD, PhD For entire Editorial Board visit : University of California, Los http://www.surgicalneurologyint.com Angeles, CA, USA Review Article Stuck at the bench: Potential natural neuroprotective compounds for concussion Anthony L. Petraglia, Ethan A. Winkler1, Julian E. Bailes2 Department of Neurosurgery, University of Rochester Medical Center, Rochester, NY, 1University of Rochester School of Medicine and Dentistry, Rochester, NY, 2Department of Neurosurgery, North Shore University Health System, Evanston, IL, USA E-mail: *Anthony L. Petraglia - [email protected]; Ethan A. Winkler - [email protected]; Julian E. Bailes - [email protected] *Corresponding author Received: 29 August 11 Accepted: 22 September 11 Published: 12 October 11 This article may be cited as: Petraglia AL, Winkler EA, Bailes JE. Stuck at the bench: Potential natural neuroprotective compounds for concussion. Surg Neurol Int 2011;2:146. Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2011/2/1/146/85987 Copyright: © 2011 Petraglia AL. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Background: While numerous laboratory studies have searched for neuroprotective treatment approaches to traumatic brain injury, no therapies have successfully translated from the bench to the bedside. Concussion is a unique form of brain injury, in that the current mainstay of treatment focuses on both physical and cognitive rest. Treatments for concussion are lacking. The concept of neuro-prophylactic compounds or supplements is also an intriguing one, especially as we are learning more about the relationship of numerous sub-concussive blows and/or repetitive concussive impacts and the development of chronic neurodegenerative disease. -

Cocrystal Prediction Using Machine Learning Models and Descriptors
applied sciences Article Cocrystal Prediction Using Machine Learning Models and Descriptors Medard Edmund Mswahili 1,† , Min-Jeong Lee 2,†, Gati Lother Martin 1, Junghyun Kim 3, Paul Kim 4, Guang J. Choi 2 and Young-Seob Jeong 1,* 1 Department of ICT Convergence, Soonchunhyang University, Asan-si 31538, Korea; [email protected] (M.E.M.); [email protected] (G.L.M.) 2 Department of Pharmaceutical Engineering, Soonchunhyang University, Asan-si 31538, Korea; [email protected] (M.-J.L.); [email protected] (G.J.C.) 3 Department of Future Convergence Technology, Soonchunhyang University, Asan-si 31538, Korea; [email protected] 4 Department of Medical Science, Soonchunhyang University, Asan-si 31538, Korea; [email protected] * Correspondence: [email protected] † These authors contributed equally to this work. Abstract: Cocrystals are of much interest in industrial application as well as academic research, and screening of suitable coformers for active pharmaceutical ingredients is the most crucial and challeng- ing step in cocrystal development. Recently, machine learning techniques are attracting researchers in many fields including pharmaceutical research such as quantitative structure-activity/property relationship. In this paper, we develop machine learning models to predict cocrystal formation. We extract descriptor values from simplified molecular-input line-entry system (SMILES) of compounds and compare the machine learning models by experiments with our collected data of 1476 instances. As a result, we found that artificial neural network shows great potential as it has the best accuracy, sensitivity, and F1 score. We also found that the model achieved comparable performance with about Citation: Mswahili, M.E.; Lee, M.-J.; half of the descriptors chosen by feature selection algorithms. -

Investigation of Carbamazepine- Nicotinamide Cocrystal Solubility and Dissolution by a UV Imaging System
Investigation of Carbamazepine- Nicotinamide cocrystal solubility and dissolution by a UV imaging system PhD Thesis Ning Qiao This thesis is submitted in partial fulfilment of the requirements of De Montfort University for the award of Doctor of Philosophy April 2014 Faculty of Health and Life Sciences De Montfort University Leicester CONTENTS CONTENTS CONTENTS ................................................................................................................................... I DECLARATION ........................................................................................................................... v ABSTRACT .................................................................................................................................. vi ACKNOWLEDGEMENTS ......................................................................................................... vii PUBLICATIONS ........................................................................................................................ viii LIST OF FIGURES ....................................................................................................................... x LIST OF TABLES ...................................................................................................................... xiii ABBREVIATIONS ..................................................................................................................... xv Chapter 1 Introduction ................................................................................................................. -

Cilnidipine, but Not Amlodipine, Ameliorates Osteoporosis in Ovariectomized Hypertensive Rats Through Inhibition of the N-Type Calcium Channel
Hypertension Research (2012) 35, 77–81 & 2012 The Japanese Society of Hypertension All rights reserved 0916-9636/12 www.nature.com/hr ORIGINAL ARTICLE Cilnidipine, but not amlodipine, ameliorates osteoporosis in ovariectomized hypertensive rats through inhibition of the N-type calcium channel Hideo Shimizu1, Hironori Nakagami2, Natsuki Yasumasa2, Osako Kiomy Mariana3, Mariko Kyutoku3, Hiroshi Koriyama3, Futoshi Nakagami3, Munehisa Shimamura2, Hiromi Rakugi1 and Ryuichi Morishita3 Both osteoporosis and high blood pressure are major diseases in aging populations. Recent studies demonstrated that some antihypertensive drugs reduced the risk of bone fracture in elderly patients. Although calcium channel blockers (CCB) are widely used as first-line antihypertensive agents, there is no evidence that they prevent osteoporosis. In this study, we investigated the effects of two types of CCB on bone metabolism: cilnidipine (L-/N-type CCB), which suppresses norepinephrine release from the sympathetic nerve, and amlodipine (L-type CCB). In ovariectomized female spontaneous hypertensive rats, administration of cilnidipine, but not amlodipine, resulted in a significant increase in the ratio of alkaline phosphatase to tartrate-resistant acid phosphatase (TRAP) and a decrease in the number of osteoclasts, as assessed by TRAP staining in the proximal tibia. Bone mineral density, moreover, was significantly higher in the cilnidipine group as compared with the amlodipine group and was associated with a significant decrease in a urinary collagen degradation product (deoxypyridinoline). The degree of prevention of osteoporosis by cilnidipine was similar to that of carvedilol (a b-blocker) because b-blockers reduce fracture risks though the inhibition of osteoclast activation. Interestingly, these effects cannot be attributed to the reduction of blood pressure because all three drugs significantly decreased blood pressure. -

Cocrystal Prediction by Artificial Neural Networks
Cocrystal prediction by artificial neural networks Jan-Joris Devogelaer, Hugo Meekes, Paul Tinnemans, Elias Vlieg, and René de Gelder* Radboud University, Institute for Molecules and Materials, Heyendaalseweg 135, 6525 AJ Nijmegen, The Netherlands E-mail: [email protected] Abstract A significant amount of attention has been given to the design and synthesis of cocrys- tals by both industry and academia because of its potential to change a molecule’s physico- chemical properties. Yet, difficulties arise when searching for adequate combinations of molecules (or coformers) to form cocrystals, hampering the efficient exploration of the tar- get’s solid-state landscape. This paper reports on the application of a data-driven cocrystal prediction method, based on two types of artificial neural network models and cocrystal data present in the Cambridge Structural Database. The models accept pairs of coformers and predict whether a cocrystal is likely to form. By combining the output of multiple models of both types, our approach shows to have excellent performance on the proposed cocrystal training and validation sets, and has an estimated accuracy of 80% for molecules for which previous cocrystallization data is unavailable. 1 1 Introduction Molecular solids appear in many different ways, and the solid-state landscape of a molecule may cover various crystalline forms, ranging from polymorphs and hydrates to more complex multicomponent crystals [1, 2]. The latter have been promoted as excellent tools to modify the physico-chemical characteristics of a target compound, such as the (aqueous) solubility, bio- availability, density, and melting point [3–8]. Multicomponent crystals therefore play a pivotal role in the effective formulation of pharmaceuticals and will continue to be part of the solid form screening process in drug development. -

Synthesis, Crystal Structure, and Solubility Analysis of a Famotidine Cocrystal
crystals Article Synthesis, Crystal Structure, and Solubility Analysis of a Famotidine Cocrystal Yan Zhang 1,2,* , Zhao Yang 2, Shuaihua Zhang 3 and Xingtong Zhou 2 1 School of Medicine and Pharmacy, Ocean University of China, Qingdao 266003, China 2 Qingdao Institute for Food and Drug Control, Qingdao 266071, China 3 School of Pharmacy, Qingdao University, Qingdao 266071, China * Correspondence: [email protected]; Tel.: +86-532-5875-9178 Received: 15 May 2019; Accepted: 10 July 2019; Published: 15 July 2019 Abstract: A novel cocrystal of the potent H2 receptor antagonist famotidine (FMT) was synthesized with malonic acid (MAL) to enhance its solubility. The cocrystal structure was characterized by X-ray single crystal diffraction, and the asymmetry unit contains one FMT and one MAL connected via intermolecular hydrogen bonds. The crystal structure is monoclinic with a P21/n space group and unit cell parameters a = 7.0748 (3) Å, b = 26.6502 (9) Å, c = 9.9823 (4) Å, α = 90, β = 104.2228 (12), γ = 90,V = 1824.42 (12) Å3, and Z = 4. The cocrystal had unique thermal, spectroscopic, and powder X-ray diffraction (PXRD) properties that differed from FMT. The solubility of the famotidine-malonic acid cocrystal (FMT-MAL) was 4.2-fold higher than FMT; the FAM-MAL had no change in FMT stability at high temperature, high humidity, or with illumination. Keywords: cocrystal; famotidine; malonic acid; crystal structure; solubility 1. Introduction Drugs with low water solubility usually show dissolution-limited absorption and low bioavailability [1]. Recent estimates suggest that approximately 40% of currently marketed drugs and up to 75% of compounds currently under development are poorly water soluble; thus, enhancing the aqueous solubility of poorly water-soluble active pharmaceutical ingredients (APIs) is a key challenge for pharmaceutical scientists [2].