Viewing (See Video Data Collection)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Heart Rate Information Sheet Finding Your Pulse Taking Your Pulse
Heart rate In this activity you will measure your heart rate and investigate the effect that other things have on your heart rate. Information sheet You can measure your heart (pulse) rate anywhere on your body where a major artery is close to the surface of your skin. The easiest places are: • on the front of your forearm • just above your wrist on your thumb side • on the side of your neck about half way between your chin and your ear. Finding your pulse Try to locate your pulse in one of these places, using the tips of your index and middle fingers. You should feel a gentle, regular beat. This is your heart rate. Do not use your thumb, as your thumb has a pulse of its own. Taking your pulse When you find your pulse, use a stopwatch or a watch with a second hand to count how many beats there are in a full minute (60 seconds). In most situations, taking your pulse rate over one minute will give a reasonably accurate result, but if you want to take your pulse after exercise, you should do so over a much shorter time interval. After exercise your pulse rate will be changing rapidly. To get a reasonably accurate result, start to measure the rate immediately after the exercise and count the number of beats in 10 seconds. Multiply this number by six to find your heart rate. For an adult, a normal resting heart rate is between 60–100 beats a minute. The fitter you are, the lower your resting heart beat will be. -

Heart to Heart - STEAM Activity
Heart to Heart - STEAM Activity Purpose: The main objective of this exercise is to introduce how the human heart works. This lesson will be divided into four different lessons, including an introduction to heart anatomy, heart beats and pulses, and the circulatory system. We will be using coloring activities, stethoscopes, and handmade pumps to reinforce the concepts seen in this lesson. Vocabulary ● Artery: A blood vessel that carries blood high in oxygen content away from the heart to the farthest reaches of the body. ● Vein: a Blood vessel that carries blood low in oxygen content from the body back to the heart. ● Atrium: One of the two upper cavities of the heart that passes blood to the ventricles. ● Ventricles: One of the two lower chambers of the heart that receives blood from the atria. ● Valves: Tissue-paper thin membranes attached to the heart wall that constantly open and close to regulate blood flow. ● Pulse: A rhythmical, mechanical throbbing of the arteries as blood pumps through them. ● Heart Rate: The number of times per minute that the heart contracts - the number of heart beats per minute (bpm). ● Taquicardia: A high resting heart rate that is usually higher than 100 beats per minute. ● Bradycardia: A low resting heart rate that is usually lower than 60 beats per minute. ● Circulation: The movement of blood through the vessels of the body by the pumping action of the heart. It distributes nutrients and oxygen and removes waste products from all parts of the body. ● Pulmonary Circulation: The portion of the circulatory system that carries deoxygenated blood from the heart to the lungs and oxygenated blood back to the heart. -

Blood Vessels: Part A
Chapter 19 The Cardiovascular System: Blood Vessels: Part A Blood Vessels • Delivery system of dynamic structures that begins and ends at heart – Arteries: carry blood away from heart; oxygenated except for pulmonary circulation and umbilical vessels of fetus – Capillaries: contact tissue cells; directly serve cellular needs – Veins: carry blood toward heart Structure of Blood Vessel Walls • Lumen – Central blood-containing space • Three wall layers in arteries and veins – Tunica intima, tunica media, and tunica externa • Capillaries – Endothelium with sparse basal lamina Tunics • Tunica intima – Endothelium lines lumen of all vessels • Continuous with endocardium • Slick surface reduces friction – Subendothelial layer in vessels larger than 1 mm; connective tissue basement membrane Tunics • Tunica media – Smooth muscle and sheets of elastin – Sympathetic vasomotor nerve fibers control vasoconstriction and vasodilation of vessels • Influence blood flow and blood pressure Tunics • Tunica externa (tunica adventitia) – Collagen fibers protect and reinforce; anchor to surrounding structures – Contains nerve fibers, lymphatic vessels – Vasa vasorum of larger vessels nourishes external layer Blood Vessels • Vessels vary in length, diameter, wall thickness, tissue makeup • See figure 19.2 for interaction with lymphatic vessels Arterial System: Elastic Arteries • Large thick-walled arteries with elastin in all three tunics • Aorta and its major branches • Large lumen offers low resistance • Inactive in vasoconstriction • Act as pressure reservoirs—expand -

Toolbox-Talks--Blood-Pressure.Pdf
TOOLBOX Toolbox Talk #1 TALKS Blood Pressure vs. Heart Rate While your blood pressure is the force of your blood moving through your blood vessels, your heart rate is the number of times your heart beats per minute. They are two separate measurements and indicators of health. • For people with high blood pressure (HBP or hypertension), there’s no substitute for measuring blood pressure. • Heart rate and blood pressure do not necessarily increase at the same rate. A rising heart rate does not cause your blood pressure to increase at the same Quarter: rate. Even though your heart is beating more times a minute, healthy blood BLOOD vessels dilate (get larger) to allow more blood to flow through more easily. PRESSURE When you exercise, your heart speeds up so more blood can reach your muscles. It may be possible for your heart rate to double safely, while your blood pressure may respond by only increasing a modest amount. Talk Number: Heart Rate and Exercise 1 In discussions about high blood pressure, you will often see heart rate Blood mentioned in relation to exercise. Your target heart rate is based on age and Pressure can help you monitor the intensity of your exercise. vs. • If you measure your heart rate (take your pulse) before, during and after Heart Rate physical activity, you’ll notice it will increase over the course of the exercise. • The greater the intensity of the exercise, the more your heart rate will increase. • When you stop exercising, your heart rate does not immediately return to your normal (resting) heart rate. -

The Icefish (Chionodraco Hamatus)
The Journal of Experimental Biology 207, 3855-3864 3855 Published by The Company of Biologists 2004 doi:10.1242/jeb.01180 No hemoglobin but NO: the icefish (Chionodraco hamatus) heart as a paradigm D. Pellegrino1,2, C. A. Palmerini3 and B. Tota2,4,* Departments of 1Pharmaco-Biology and 2Cellular Biology, University of Calabria, 87030, Arcavacata di Rende, CS, Italy, 3Department of Cellular and Molecular Biology, University of Perugia, 06126, Perugia, Italy and 4Zoological Station ‘A. Dohrn’, Villa Comunale, 80121, Napoli, Italy *Author for correspondence (e-mail: [email protected]) Accepted 13 July 2004 Summary The role of nitric oxide (NO) in cardio-vascular therefore demonstrate that under basal working homeostasis is now known to include allosteric redox conditions the icefish heart is under the tonic influence modulation of cell respiration. An interesting animal for of a NO-cGMP-mediated positive inotropism. We also the study of this wide-ranging influence of NO is the cold- show that the working heart, which has intracardiac adapted Antarctic icefish Chionodraco hamatus, which is NOS (shown by NADPH-diaphorase activity and characterised by evolutionary loss of hemoglobin and immunolocalization), can produce and release NO, as multiple cardio-circulatory and subcellular compensations measured by nitrite appearance in the cardiac effluent. for efficient oxygen delivery. Using an isolated, perfused These results indicate the presence of a functional NOS working heart preparation of C. hamatus, we show that system in the icefish heart, possibly serving a both endogenous (L-arginine) and exogenous (SIN-1 in paracrine/autocrine regulatory role. presence of SOD) NO-donors as well as the guanylate cyclase (GC) donor 8Br-cGMP elicit positive inotropism, while both nitric oxide synthase (NOS) and sGC Key words: nitric oxide, heart, Antarctic teleost, icefish, Chionodraco inhibitors, i.e. -

Your Pulse and Target Heart Rate
Skip to main content ×Close Menu We use cookies to improve your experience on our website. By closing this banner or interacting with our site, you acknowledge and agree to this. Legal Notices MobileClose Menu Find A Doctor Your Visit Pay a Bill Log in to MyGillette Learn more about MyGillette Patient Education Patient Services and Resources Your Rights and Medical Records Understanding Costs, Insurance and the Gillette Assistance Programs Services to Support Your Family Interpreter Services International Patients Parent Resources and Support Prepare for Your Visit Prepare for Clinic Visits and Tests Prepare for Surgery Take a Hospital Tour Transfer Your Medical Records Transportation and Accommodations Ways to Prepare and Comfort Your Child During Your Visit or Hospital Stay Commitment to a Safe and Healing Environment Our Hospital Units Your Hospital Room St. Paul Campus Amenities and Activities Visit or Contact a Patient Conditions & Care All Conditions Cerebral Palsy Neuromuscular Disorders Scoliosis Craniosynostosis Brain Injury All Tests & Treatments Shunt Surgery for Hydrocephalus Gait and Motion Analysis Selective Dorsal Rhizotomy (SDR) Gillette Craniocap© Orthosis Botulinum Toxin and Phenol (Injected Spasticity Medications) All Specialties & Services Orthopedics Rehabilitation Services Craniofacial and Plastic Surgery Neurology Neurosurgery Virtual Visits Pediatric Expert Consults Virtual Rehab Therapy Sleep Medicine Virtual Care Get Involved Advocating for Your Family Community Health United Cerebral Palsy of MN Donors & Supporters -

The Heart Institute
The Heart Institute Division of Cardiology 4650 Sunset Blvd., #34, Los Angeles, CA 90027 Phone: 323-361-2461 Fax: 323-361-1513 The Heart Institute at Children’s Hospital Los Angeles is CHLA.org/CARDIOLOGY one of the top-ranked pediatric heart programs in the Division of nation—with a long history of exceptional and innovative Cardiothoracic Surgery 4650 Sunset Blvd., #66 care for the most complex pediatric cardiac conditions. Los Angeles, CA 90027 Phone: 323-361-4148 Fax: 323-361-3668 We treat patients from fetus to adulthood and serve as CHLA.org/CTSurgery a major tertiary referral center for all forms of congenital Referrals Phone: 888-631-2452 and acquired heart disease. Fax: 323-361-8988 Email: [email protected] Physician Portal: https://myCHLA.CHLA.org We offer an integrated inpatient and outpatient Our Cardiothoracic Intensive Care Unit (CTICU) complement of services that brings together experts was the first of its kind on the West Coast, using in cardiology, cardiothoracic surgery, cardiothoracic innovative treatments including extracorporeal transplant, cardiothoracic intensive care and membrane oxygenation (ECMO) and ventricular cardiovascular acute care in a centrally located, assist services. state-of-the-art healing environment. With fewer steps to navigate, our patients and families receive care Our two state-of-the-art catheterization laboratories that is more streamlined and less stressful. use the latest technology to provide accurate cardiac data while reducing radiation. Our programmatic emphasis on high-complexity surgeries in neonates has produced outcomes that are among the best in the country, as shown in the most recent Society of Thoracic Surgeons report. -

A Review of the Stroke Volume Response to Upright Exercise in Healthy Subjects
190 Br J Sports Med: first published as 10.1136/bjsm.2004.013037 on 25 March 2005. Downloaded from REVIEW A review of the stroke volume response to upright exercise in healthy subjects C A Vella, R A Robergs ............................................................................................................................... Br J Sports Med 2005;39:190–195. doi: 10.1136/bjsm.2004.013037 Traditionally, it has been accepted that, during incremental well trained athletes (subject sex was not stated). Unfortunately, these findings were exercise, stroke volume plateaus at 40% of VO2MAX. largely ignored and it became accepted that However, recent research has documented that stroke stroke volume plateaus during exercise of increasing intensity. volume progressively increases to VO2MAX in both trained More recent investigations have reported that and untrained subjects. The stroke volume response to stroke volume progressively increases in certain 7–12 incremental exercise to VO2MAX may be influenced by people. The mechanisms for the continual training status, age, and sex. For endurance trained increase in stroke volume are not completely understood. Gledhill et al7 proposed that subjects, the proposed mechanisms for the progressive enhanced diastolic filling and subsequent increase in stroke volume to VO2MAX are enhanced diastolic enhanced contractility are responsible for the filling, enhanced contractility, larger blood volume, and increased stroke volume in trained subjects. However, an increase in stroke volume with an decreased cardiac afterload. For untrained subjects, it has increase in exercise intensity has also been been proposed that continued increases in stroke volume reported in untrained subjects.89Table 1 presents may result from a naturally occurring high blood volume. a summary of the past research that has quantified stroke volume during exercise. -

Effects of Vasodilation and Arterial Resistance on Cardiac Output Aliya Siddiqui Department of Biotechnology, Chaitanya P.G
& Experim l e ca n i t in a l l C Aliya, J Clinic Experiment Cardiol 2011, 2:11 C f a Journal of Clinical & Experimental o r d l DOI: 10.4172/2155-9880.1000170 i a o n l o r g u y o J Cardiology ISSN: 2155-9880 Review Article Open Access Effects of Vasodilation and Arterial Resistance on Cardiac Output Aliya Siddiqui Department of Biotechnology, Chaitanya P.G. College, Kakatiya University, Warangal, India Abstract Heart is one of the most important organs present in human body which pumps blood throughout the body using blood vessels. With each heartbeat, blood is sent throughout the body, carrying oxygen and nutrients to all the cells in body. The cardiac cycle is the sequence of events that occurs when the heart beats. Blood pressure is maximum during systole, when the heart is pushing and minimum during diastole, when the heart is relaxed. Vasodilation caused by relaxation of smooth muscle cells in arteries causes an increase in blood flow. When blood vessels dilate, the blood flow is increased due to a decrease in vascular resistance. Therefore, dilation of arteries and arterioles leads to an immediate decrease in arterial blood pressure and heart rate. Cardiac output is the amount of blood ejected by the left ventricle in one minute. Cardiac output (CO) is the volume of blood being pumped by the heart, by left ventricle in the time interval of one minute. The effects of vasodilation, how the blood quantity increases and decreases along with the blood flow and the arterial blood flow and resistance on cardiac output is discussed in this reviewArticle. -

60 Arterial Pressure–Based Cardiac Output Monitoring 525
PROCEDURE Arterial Pressure–Based Cardiac 60 Output Monitoring Susan Scott PURPOSE: Arterial pressure–based cardiac output monitoring is a minimally invasive technology that can be used to obtain hemodynamic data on a continuous basis. PREREQUISITE NURSING • The difference between the systolic and diastolic pressures KNOWLEDGE is called the pulse pressure, with a normal value of 40 mm Hg. • Arterial pressure is determined by the relationship between • Knowledge of the anatomy and physiology of the cardio- blood fl ow through the vessels (cardiac output), the com- vascular system is necessary. pliance of the aorta and larger vessels, and the resistance • Knowledge of the anatomy and physiology of the vascu- of the more peripheral vessel walls (systemic vascular lature and adjacent structures is needed. resistance). The arterial pressure is therefore affected by • Understanding of the pathophysiologic changes that occur any factors that change either cardiac output, compliance, in heart disease and affect fl ow dynamics is necessary. or systemic vascular resistance. • Understanding of aseptic technique is needed. • The average arterial pressure during a cardiac cycle is • Understanding of the hemodynamic effects of vasoactive called the mean arterial pressure (MAP). It is not the medications and fl uid resuscitation is needed. average of the systolic plus the diastolic pressures, because 1 • Understanding of the principles involved in hemodynamic at normal heart rates systole accounts for 3 of the cardiac 2 monitoring is necessary. cycle and diastole accounts for 3 of the cardiac cycle. • Knowledge of invasive cardiac output monitoring is needed. The MAP is calculated automatically by most patient • Knowledge of arterial waveform interpretation is needed. -

Jugular Venous Pressure
NURSING Jugular Venous Pressure: Measuring PRACTICE & SKILL What is Measuring Jugular Venous Pressure? Measuring jugular venous pressure (JVP) is a noninvasive physical examination technique used to indirectly measure central venous pressure(i.e., the pressure of the blood in the superior and inferior vena cava close to the right atrium). It is a part of a complete cardiovascular assessment. (For more information on cardiovascular assessment in adults, see Nursing Practice & Skill ... Physical Assessment: Performing a Cardiovascular Assessment in Adults ) › What: Measuring JVP is a screening mechanism to identify abnormalities in venous return, blood volume, and right heart hemodynamics › How: JVP is determined by measuring the vertical distance between the sternal angle and the highest point of the visible venous pulsation in the internal jugular vein orthe height of the column of blood in the external jugular vein › Where: JVP can be measured in inpatient, outpatient, and residential settings › Who: Nurses, nurse practitioners, physician assistants, and treating clinicians can measure JVP as part of a complete cardiovascular assessment What is the Desired Outcome of Measuring Jugular Venous Pressure? › The desired outcome of measuring JVP is to establish the patient’s JVP within the normal range or for abnormal JVP to be identified so that appropriate treatment may be initiated. Patients’ level of activity should not be affected by having had the JVP measured ICD-9 Why is Measuring Jugular Venous Pressure Important? 89.62 › The JVP is -
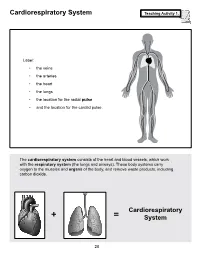
Cardiorespiratory System Teaching Activity 1
Cardiorespiratory System Teaching Activity 1. Label: • the veins • the arteries • the heart • the lungs • the location for the radial pulse • and the location for the carotid pulse. The cardiorespiratory system consists of the heart and blood vessels, which work with the respiratory system (the lungs and airways). These body systems carry oxygen to the muscles and organs of the body, and remove waste products, including carbon dioxide. Cardiorespiratory + = System 28 The Most Important Muscle: The Heart Teaching Activity 1, continued. In the circulatory system, the heart is a muscle that acts as a pump. In fact, the heart is a double pump. Blood that needs oxygen enters the heart and is pumped by the first pump to the lungs. The second pump of the heart pumps the oxygen-rich blood to all the other parts of the body. This gives the heart its common, “lub-dub” sound. The number of times the heart pumps, or beats, is counted in a minute. This is known as the pulse rate. A person’s pulse is affected by their current level of activity. If you are sleeping or do no physical activity at all, your heart is pumping at a resting heart rate. When active, you are using all your body systems. These systems require fuel in the form of calories (found in food), and oxygen (what you breathe). The more active you are, the Hearty Facts more fuel your muscles need. This is why your breathing rate and your heart rate increase when • Your system of blood vessels (arteries, you exercise. veins, and capillaries) is over 60,000 miles long.