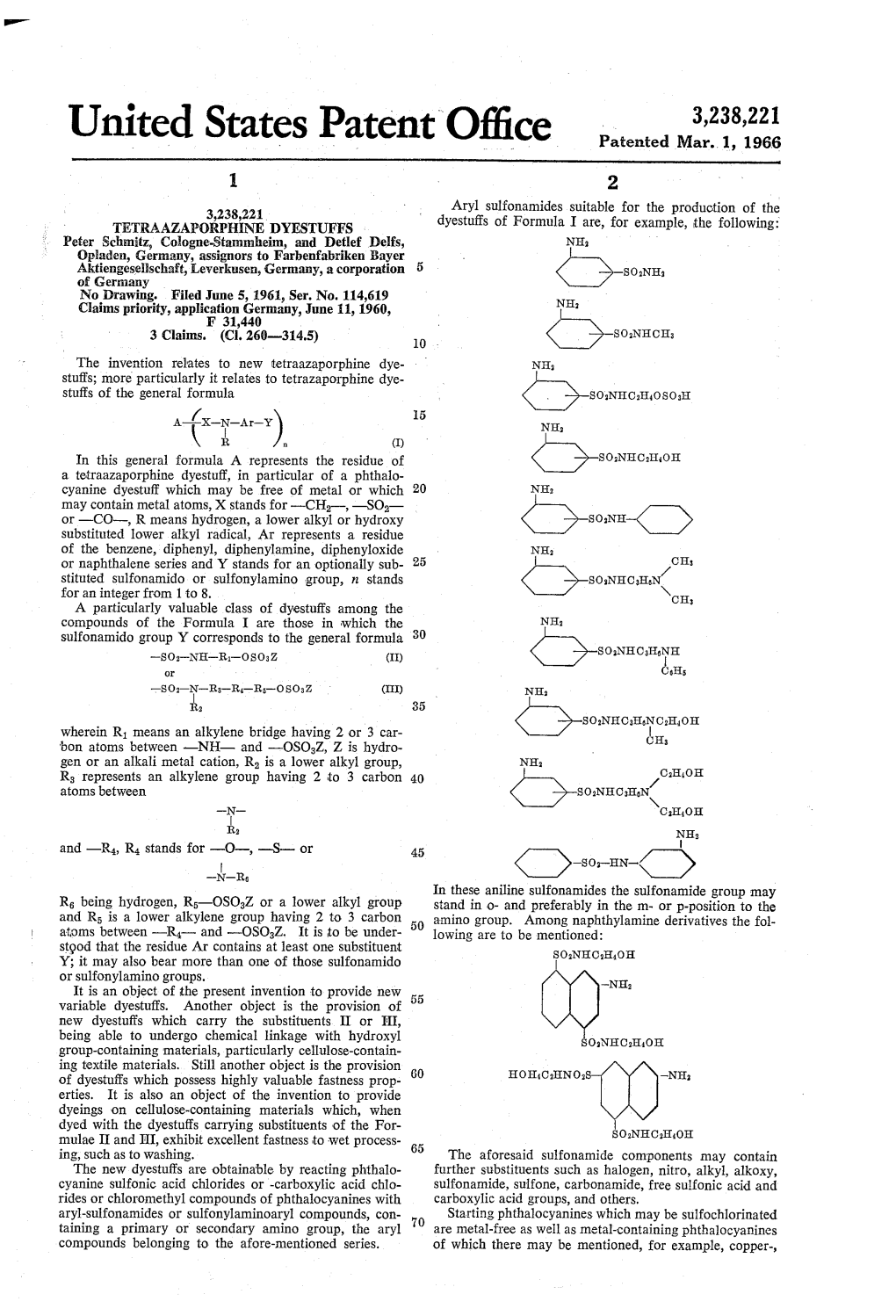
United States Patent Office Patented Mar
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Harmonized Tariff Schedule of the United States (2020) Revision 14 Annotated for Statistical Reporting Purposes
Harmonized Tariff Schedule of the United States (2020) Revision 14 Annotated for Statistical Reporting Purposes SECTION VI PRODUCTS OF THE CHEMICAL OR ALLIED INDUSTRIES VI-1 Notes 1. (a) Goods (other than radioactive ores) answering to a description in heading 2844 or 2845 are to be classified in those headings and in no other heading of the tariff schedule. (b) Subject to paragraph (a) above, goods answering to a description in heading 2843, 2846 or 2852 are to be classified in those headings and in no other heading of this section. 2. Subject to note 1 above, goods classifiable in heading 3004, 3005, 3006, 3212, 3303, 3304, 3305, 3306, 3307, 3506, 3707 or 3808 by reason of being put up in measured doses or for retail sale are to be classified in those headings and in no other heading of the tariff schedule. 3. Goods put up in sets consisting of two or more separate constituents, some or all of which fall in this section and are intended to be mixed together to obtain a product of section VI or VII, are to be classified in the heading appropriate to that product, provided that the constituents are: (a) Having regard to the manner in which they are put up, clearly identifiable as being intended to be used together without first being repacked; (b) Entered together; and (c) Identifiable, whether by their nature or by the relative proportions in which they are present, as being complementary one to another. Additional U.S. Notes 1. In determining the amount of duty applicable to a solution of a single compound in water subject to duty in this section at a specific rate, an allowance in weight or volume, as the case may be, shall be made for the water in excess of any water of crystallization which may be present in the undissolved compound. -

Mechanical Seals Conventional and Gas-Lubricated for Pumps, Compressors, Agitators and Similar Applications
® Mechanical Seals Conventional and Gas-lubricated for Pumps, Compressors, Agitators and Similar Applications. Seal Supply Systems and Components. Design Manual 15.3 BURGMANNinyourvicinity ServiceCenterOst KE-BurgmannU.K. INDIA MYANMAR TAIWAN Germany AirparkMerseburg EatonBank,Congleton OutsideEurope BurgmannIndiaPVT.LTD. OneCo.Ltd. FriendlyTradingCo.,Inc. Hans-Grade-Straße4 GB-CheshireSW121NZ A.K.IndustrialEstate No.22,7Mile,PyayRd., 11Fl.-1,No.1PaoSenRoad Kundenbetreuungs-Center D-06217Merseburg Phone+44/1260/291289 ARGENTINA MadinaManzilCompound MayangoneTownship YongHoCity,TaipeiHsien Nord/WerksbüroBremen Phone+49/3461/842920 Telefax+44/1260/291303 ChilicoteS.A. S.V.Road,Goregaon(West) Yangon,Myanmar Taiwan/R.O.C. SinstorferKirchweg74- 92 Telefax+49/3461/842930 Avda.JulioA.Roca546 Bombay400062 Phone+951244969 Phone+886/22/9279070 D-21 077Hamburg GREECE Phone+91/22/8741534 Telefax+951650469 Telefax+886/22/9279377 ServiceCenterRhein-Main C1067ABNBuenosAires,Argentina Phone+49/421/562375 A.G.Stambolidis&Co. Telefax+91/22/8748810 ImWinkel4 Phone+54/11/43438469 NEWZEALAND THAILAND Telefax+49/421/563396 Mitrodorou22 Telefax+54/11/43314278 BranchOffices:Bangalore,Baroda, D-67547Worms GR-10441Athen Chennay,Cochin,Delhi,Hyderabad, BurgmannSealsNewZealand BurgmannThailandCo.Ltd. Kundenbetreuungs-Center Phone+49/6241/932520 Phone+30/21/05150665 AUSTRALIA Kota,Panvel,Pune,Visakhapatnam, UnitF14ClemwayPlace BangaTowerC,Floor18th Ost/WerksbüroCottbus Telefax+49/6241/932531 Telefax+30/21/05150810 BurgmannSeals Mumbai,Surat. Henderson,Auckland 40/14Moo12Banga-TradRd. -

(12) Patent Application Publication (10) Pub. No.: US 2002/0161255A1 Ishii Et Al
US 2002O161255A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2002/0161255A1 Ishii et al. (43) Pub. Date: Oct. 31, 2002 (54) PROCESS FOR PREPARING ORGANIC (52) U.S. Cl. ................................................. 558/40; 562/95 SULFURACDS OR SALTS THEREOF (76) Inventors: Yasutaka Ishii, Takatsuki-shi (JP); Tatsuya Nakano, Hyogo (JP) (57) ABSTRACT Correspondence Address: BRCH STEWART KOLASCH & BRCH A process for producing organic Sulfur acid or a Salt thereof PO BOX 747 of the present invention allows an organic Substrate to react FALLS CHURCH, VA 22040-0747 (US) with a Sulfur oxide in the presence of a metallic compound catalyst and in the absence of N-hydroxy and N-oxo cyclic (21) Appl. No.: 09/959,648 imide compounds and thereby yields a corresponding organic Sulfur acid or a Salt thereof. Such organic Substrates (22) PCT Filed: Feb. 27, 2001 include, for example, (a) homocyclic or heterocyclic com pounds having a methylene group, (b) compounds having a (86) PCT No.: PCT/JP01/01462 methine carbon atom, (c) compounds having a methyl group (30) Foreign Application Priority Data or methylene group at the adjacent position to an unsaturated bond, (d) non-aromatic heterocyclic compounds having a Mar. 2, 2000 (JP)....................................... O58055/2000 carbon-hydrogen bond at the adjacent position to a hetero atom, and (e) straight-chain alkanes. The Sulfur oxide Publication Classification includes, for example, Sulfur dioxide. This production pro ceSS can efficiently produce an organic Sulfur acid or a Salt (51) Int. Cl." ........................... C07C 35/02; CO7C 33/04 thereof under mild conditions. US 2002/0161255 A1 Oct. -

6 Chemical Skin Burns
53 6 Chemical Skin Burns Magnus Bruze, Birgitta Gruvberger, Sigfrid Fregert Contents aged to a point where there is no return to viability; in other words, a necrosis develops [7, 43, 45]. One 6.1 Introduction . 53 single skin exposure to certain chemicals can result 6.2 Definition . 53 in a chemical burn. These chemicals react with intra- 6.3 Diagnosis . 56 and intercellular components in the skin. However, 6.4 Clinical Features . 56 the action of toxic (irritant) chemicals varies caus- 6.5 Treatment . 57 ing partly different irritant reactions morphologically. 6.6 Complications . 58 They can damage the horny layer, cell membranes, 6.7 Prevention . 59 6.8 Summary . 59 lysosomes, mast cells, leukocytes, DNA synthesis, References . 60 blood vessels, enzyme systems, and metabolism. The corrosive action of chemicals depends on their chem- ical properties, concentration, pH, alkalinity, acidity, temperature, lipid/water solubility, interaction with 6.1 Introduction other substances, and duration and type (for exam- ple, occlusion) of skin contact. It also depends on the Chemical skin burns are particularly common in in- body region, previous skin damage, and possibly on dustry, but they also occur in non-work-related en- individual resistance capacity. vironments. Occupationally induced chemical burns Many substances cause chemical burns only when are frequently noticed when visiting and examining they are applied under occlusion from, for example, workers at their work sites. Corrosive chemicals used gloves, boots, shoes, clothes, caps, face masks, ad- in hobbies are an increasing cause of skin burns. Dis- hesive plasters, and rings. Skin folds may be formed infectants and cleansers are examples of household and act occlusively in certain body regions, e.g., un- products which can cause chemical burns. -

List of Chemical Resistance of Materials in Contact with Liquid ANALYSETTE 22 Next & ANALYSETTE 28 Imagesizer LASER PARTICLE SIZER & PARTICLE SIZER
List of chemical resistance of materials in contact with liquid ANALYSETTE 22 NeXT & ANALYSETTE 28 ImageSizer LASER PARTICLE SIZER & PARTICLE SIZER This chart information is based on FRITSCH's knowledge and experience and are intended as a guide and not as a guarantee. We cannot assure the perfect performance of the materials as that depends on many factors as working pressure, pressure picks, fluid, ambient temperature, fluid temperature, concentra‐ tion, duration of exposure, etc… Fritsch GmbH Milling and Sizing Industriestrasse 8 D - 55743 Idar-Oberstein Telephone: +49 (0) 6784/ 70-0 Email: [email protected] Internet: www.fritsch.de Version 04/2020 Index 001 Table of contents Table of contents 1 Materials in contact with liquid.................................................... 4 2 Abbreviations................................................................................. 5 3 List of chemical resistance............................................................. 7 3.1 KEY......................................................................................... 7 3.2 Note....................................................................................... 7 3.3 Table 1.................................................................................... 7 3.4 Table 2.................................................................................. 97 3.5 Table 3................................................................................ 105 - 3 - Materials in contact with liquid 1 Materials in contact with liquid Wet dispersion -

FOR CHLOROSULFONIC ACID (CSA) (CAS Reg
ACUTE EXPOSURE GUIDELINE LEVELS (AEGLs) FOR CHLOROSULFONIC ACID (CSA) (CAS Reg. No. 7790-94-5) INTERIM CHLOROSULFONIC ACID (CSA) INTERIM: 06/2008 (Page 2 of 33) 1 PREFACE 2 3 Under the authority of the Federal Advisory Committee Act (FACA) P. L. 92-463 of 4 1972, the National Advisory Committee for Acute Exposure Guideline Levels for Hazardous 5 Substances (NAC/AEGL Committee) has been established to identify, review and interpret 6 relevant toxicologic and other scientific data and develop AEGLs for high priority, acutely toxic 7 chemicals. 8 9 AEGLs represent threshold exposure limits for the general public and are applicable to 10 emergency exposure periods ranging from 10 minutes to 8 hours. Three levels C AEGL-1, 11 AEGL-2 and AEGL-3 C are developed for each of five exposure periods (10 and 30 minutes, 1 12 hour, 4 hours, and 8 hours) and are distinguished by varying degrees of severity of toxic effects. 13 The three AEGLs are defined as follows: 14 15 AEGL-1 is the airborne concentration (expressed as parts per million or milligrams per 16 cubic meter [ppm or mg/m3]) of a substance above which it is predicted that the general 17 population, including susceptible individuals, could experience notable discomfort, irritation, or 18 certain asymptomatic, non-sensory effects. However, the effects are not disabling and are 19 transient and reversible upon cessation of exposure. 20 21 AEGL-2 is the airborne concentration (expressed as ppm or mg/m3) of a substance above 22 which it is predicted that the general population, including susceptible individuals, could 23 experience irreversible or other serious, long-lasting adverse health effects or an impaired ability 24 to escape. -

Staging Category Base Rate Article Description HTS Heading
HTS Heading/Article Subheading description Base Rate Staging Category I. CHEMICAL ELEMENTS 2801 Fluorine, chlorine, bromine and iodine: 28011000 -Chlorine Free E 28012000 -Iodine Free E 280130 -Fluorine; bromine: 28013010 --Fluorine 3.7% A 28013020 --Bromine 5.5% A 28020000 Sulfur, sublimed or precipitated; colloidal sulfur Free E Carbon (carbon blacks and other forms of carbon not 28030000 elsewhere specified or included) Free E 2804 Hydrogen, rare gases and other nonmetals: 28041000 -Hydrogen 3.7% A -Rare gases: 28042100 --Argon 3.7% A 28042900 --Other 3.7% A 28043000 -Nitrogen 3.7% A 28044000 -Oxygen 3.7% A 28045000 -Boron; tellurium Free E -Silicon: --Containing by weight not less than 99.99 percent of 28046100 silicon Free E 280469 --Other: ---Containing by weight less than 99.99 percent but not 28046910 less than 99 percent of silicon 5.3% B 28046950 ---Other 5.5% B 28047000 -Phosphorus Free E 28048000 -Arsenic Free E 28049000 -Selenium Free E Alkali or alkaline-earth metals; rare-earth metals, scandium and yttrium, whether or not intermixed or 2805 interalloyed; mercury: -Alkali metals: 28051100 --Sodium 5.3% B 28051900 --Other 5.5% B -Alkaline-earth metals: 28052100 --Calcium 3% A 280522 --Strontium and barium: 28052210 ---Strontium 3.7% A 28052220 ---Barium Free E -Rare-earth metals, scandium and yttrium, whether or 28053000 not intermixed or interalloyed 5% A 28054000 -Mercury 1.7% A II. INORGANIC ACIDS AND INORGANIC OXYGEN COMPOUNDS OF NONMETALS Hydrogen chloride (Hydrochloric acid); chlorosulfuric 2806 acid: 28061000 -Hydrogen -

(CDR) by CASRN Or Accession Number
List of Chemicals Reported for the 2012 Chemical Data Reporting (CDR) by CASRN or Accession Number For the 2012 CDR, 7,674 unique chemicals were reported by manufacturers (including importers). Chemicals are listed by CAS Registry Number (for non-confidential chemicals) or by TSCA Accession Number (for chemicals listed on the confidential portion of the TSCA Inventory). CASRN or CASRN or ACCESSION ACCESSION NUMBER CA INDEX NAME or GENERIC NAME NUMBER CA INDEX NAME or GENERIC NAME 100016 Benzenamine, 4-nitro- 10042769 Nitric acid, strontium salt (2:1) 10006287 Silicic acid (H2SiO3), potassium salt (1:2) 10043013 Sulfuric acid, aluminum salt (3:2) 1000824 Urea, N-(hydroxymethyl)- 10043115 Boron nitride (BN) 100107 Benzaldehyde, 4-(dimethylamino)- 10043353 Boric acid (H3BO3) 1001354728 4-Octanol, 3-amino- 10043524 Calcium chloride (CaCl2) 100174 Benzene, 1-methoxy-4-nitro- 100436 Pyridine, 4-ethenyl- 10017568 Ethanol, 2,2',2''-nitrilotris-, phosphate (1:?) 10043842 Phosphinic acid, manganese(2+) salt (2:1) 2,7-Anthracenedisulfonic acid, 9,10-dihydro- 100447 Benzene, (chloromethyl)- 10017591 9,10-dioxo-, sodium salt (1:?) 10045951 Nitric acid, neodymium(3+) salt (3:1) 100185 Benzene, 1,4-bis(1-methylethyl)- 100469 Benzenemethanamine 100209 1,4-Benzenedicarbonyl dichloride 100470 Benzonitrile 100210 1,4-Benzenedicarboxylic acid 100481 4-Pyridinecarbonitrile 10022318 Nitric acid, barium salt (2:1) 10048983 Phosphoric acid, barium salt (1:1) 9-Octadecenoic acid (9Z)-, 2-methylpropyl 10049044 Chlorine oxide (ClO2) 10024472 ester Phosphoric acid, -

Pdf 800.63 K
Iranian Journal of Catalysis SPOTLIGHT 3(2), 2013, 115-117 IRANIAN JOURNAL OF CATALYSIS Chlorosulfonic acid: A high potential reagent in catalyst design Compiled by Vahid Khakyzadeh* Vahid Khakyzadeh was born in Hamedan, Iran. He received his B.S. degree (Pure Chemistry) and M.S. degree (Organic Chemistry) from Bu-Ali Sina University, Hamedan, Iran, at 2008, 2010 respectively under supervision of professor Mohammad Ali Zolfigol. He is currently working on his Ph. D. under the supervision of professor Mohammad Ali Zolfigol in organic chemistry. Among his recent awards, Vahid was awarded the outstanding MSc student (2009), the outstanding PhD student (2011) and distinguished PhD student at This feature focuses on a reagent chosen Bu-Ali Sina University (2012). He has also participated in over 27 research by a postgraduate, highlighting the uses articles and 1 book. His research interests are green chemistry and catalysis, and preparation of the reagent in current including the application of homogeneous, heterogeneous catalysis in organic synthesis. research. E-mail: [email protected] Introduction Chlorosulfonic acid was first prepared by Williamson this reagent used for designing of various acidic in 1854 [1]. Chlorosulfonic acid (ClSO3H) is a catalysts. colourless or straw-coloured liquid which fumes in air and decomposes slightly at its boiling point (151- It is also used as a vulcanization accelerator, a source 152°C) [2]. It is a strong acid which is toxic and of anhydrous hydrogen chloride and in the tanning, corrosive and behaves as a dehydrating, oxidizing textile and paper industries [4]. and chlorinating agent. It is soluble in some organic Preparation solvents, such as: CHCl3, CH2Cl2, CH3COOH, acetic anhydride and nitrobenzene. -

(12) Patent Application Publication (10) Pub. No.: US 2004/0242932 A1 Earle Et Al
US 2004O242932A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0242932 A1 Earle et al. (43) Pub. Date: Dec. 2, 2004 (54) AROMATIC SULFONATION REACTIONS (30) Foreign Application Priority Data Oct. 10, 2000 (GB)......................................... OO24747.8 (76) Inventors: Martyn John Earle, Finaghy Belfast (GB); Suhas Prabhakar Katdare, Publication Classification Belfast (GB) (51) Int. Cl." ..................................................... C07C 39/02 (52) U.S. Cl. ................................................................ 564/86 Correspondence Address: DRINKER BIDDLE & REATH (57) ABSTRACT ONE LOGAN SQUARE 18TH AND CHERRY STREETS A proceSS for the Sulfonation of an aromatic compound PHILADELPHIA, PA 19103-6996 (US) wherein the aromatic compound and Sulfonating agent are admixed in the presence of an ionic liquid is described. The (21) Appl. No.: 10/398,531 method for the Sulfonation of aromatic compounds in (e.g. water stable) ionic liquids offers advantages over conven (22) PCT Filed: Oct. 5, 2001 tional Sulfonation reactions. These are that no by-products form, the ionic liquid is not consumed, and the Sulfonating (86) PCT No.: PCT/GB01/04427 agent (e.g. SO) is relatively inexpensive. US 2004/0242932 A1 Dec. 2, 2004 AROMATIC SULFONATION REACTIONS fate, or a chloride, bromide or other halide. Other anions include Sulfur containing anions including Sulfate or Sul 0001. The present invention relates to a process for the phite, hydrogenSulfate, oxoanions of metals, Selenium, tel Sulfonation of aromatic compounds. lurium, phosphorus, arsenic, antimony, and bismuth based 0002 The sulfonation of aromatic compounds can be anions. achieved by a number of methods. Classically this involves the reaction of an aromatic compound with oleum, the 0009 More than one ionic liquid or any combination of reaction with sulfur trioxide in various organic solvents. -

New York City Department of Environmental Protection Community Right-To-Know: List of Hazardous Substances
New York City Department of Environmental Protection Community Right-to-Know: List of Hazardous Substances Updated: 12/2015 Definitions SARA = The federal Superfund Amendments and Reauthorization Act (enacted in 1986). Title III of SARA, known as the Emergency Planning and Community Right-to-Act, sets requirements for hazardous chemicals, improves the public’s access to information on chemical hazards in their community, and establishes reporting responsibilities for facilities that store, use, and/or release hazardous chemicals. RQ = Reportable Quantity. An amount entered in this column indicates the substance may be reportable under §304 of SARA Title III. Amount is in pounds, a "K" represents 1,000 pounds. An asterisk following the Reporting Quantity (i.e. 5000*) will indicate that reporting of releases is not required if the diameter of the pieces of the solid metal released is equal to or exceeds 100 micrometers (0.004 inches). TPQ = Threshold Planning Quantity. An amount entered in this column reads in pounds and indicates the substance is an Extremely Hazardous Substance (EHS), and may require reporting under sections 302, 304 & 312 of SARA Title III. A TPQ with a slash (/) indicates a "split" TPQ. The number to the left of the slash is the substance's TPQ only if the substance is present in the form of a fine powder (particle size less than 100 microns), molten or in solution, or reacts with water (NFPA rating = 2, 3 or 4). The TPQ is 10,000 lb if the substance is present in other forms. A star (*) in the 313 column= The substance is reportable under §313 of SARA Title III. -

Neutralized Alkyl Ether Sulfuric Acid Half-Ester Compositions Containing
Europaisches Patentamt 19 European Patent Office Office europeen des brevets © Publication number: 0 337 406 B1 EUROPEAN PATENT SPECIFICATION @ Date of publication of patent specification © int. ci.5: C07C 305/10, A61K 7/075 02.09.92 Bulletin 92/36 © Application number: 89106434.7 © Date of filing : 11.04.89 @ Neutralized alky I ether sulfuric acid half-ester compositions containing poly hydroxy oligomers. © Priority : 12.04.88 US 180408 @ Proprietor : HENKEL CORPORATION (a Delaware corp.) 300 Brookside Avenue Ambler, PA 19002 (US) @ Date of publication of application 18.10.89 Bulletin 89/42 © Inventor : Abend, Phillip Gary 2100 Linwood Avenue © Publication of the grant of the patent : Fort Lee New Jersey 07024 (US) 02.09.92 Bulletin 92/36 © Representative : von Kreisler, Alek et al © Designated Contracting States : Patentanwalte Von AT BE CH DE ES FR GB IT LI NL SE Kreisler-Selting-Werner-Schdnwald-Fues- Dallmeyer Deichmannhaus W-5000 Koln 1 (DE) © References cited : EP-A- 0 098 632 US-A- 3 843 706 CO CO o h- co CO Note : Within nine months from the publication of the mention of the grant of the European patent, any person may give notice to the European Patent Office of opposition to the European patent granted. Notice of opposition shall be filed in a written reasoned statement. It shall not be deemed to have been LU filed until the opposition fee has been paid (Art. 99(1) European patent convention). Jouve, 18, rue Saint-Denis, 75001 PARIS EP 0 337 406 B1 Description 1. Field of the Invention: 5 Alkyl ether sulfates comprise an important class of anionic surfactants particularly extensively used in shampoos and light-duty liquid detergents.