Palaeobotanical Redux: Revisiting the Age of the Angiosperms Patrick S
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Jurassic Fossil Wood Diversity from Western Liaoning, NE China
Jiang et al. Journal of Palaeogeography (2019) 8:1 https://doi.org/10.1186/s42501-018-0018-y Journal of Palaeogeography RESEARCH Open Access The Jurassic fossil wood diversity from western Liaoning, NE China Zi-Kun Jiang1,2, Yong-Dong Wang2,3*, Ning Tian4,5, Ao-Wei Xie2,6, Wu Zhang7, Li-Qin Li2 and Min Huang1 Abstract Western Liaoning is a unique region in China that bears diverse types of Jurassic plants, including leaves, fern rhizomes, and wood, providing significant proxy for vegetation and palaeoenvironment reconstruction of the well-known Yanliao Flora in East Asia. In particular, the silicified wood is very abundant in the fossil Lagerstätte of the Jurassic Tiaojishan Formation in Beipiao, western Liaoning. Previous and recent systematic investigations documented a high diversity of the Jurassic wood assemblages. These assemblages are dominated by conifers, followed by cycads and ginkgoaleans. In total, about 30 species belonging to 21 genera of fossil wood have been recorded so far, which are represented by Cycadopsida, Ginkgopsida, Coniferopsida, and Gymnospermae incertae sedis. The evolutionary implications of several distinctive fossil wood taxa as well as palaeoclimate implications are summarized based on their anatomical structures and growth ring patterns. This work approaches the vegetation development and evolutionary significances of the wood taxa and their relatives, and provides clues for the further understanding of the diversity of the Jurassic Yanliao Flora in East Asia. Keywords: Fossil wood, Diversity, Evolution, Tiaojishan Formation, Jurassic 1 Introduction 2004;Wangetal.,2009). Among these localities, western Fossil floras are a significant record for the vegetation Liaoning is a well-known fossil Lagerstätte with diverse and for the palaeoenvironment reconstructions of the and well-preserved fossil plant foliages and wood (Zhang Mesozoic. -

A Palaeoenvironmental Reconstruction of the Middle Jurassic of Sardinia (Italy) Based on Integrated Palaeobotanical, Palynological and Lithofacies Data Assessment
Palaeobio Palaeoenv DOI 10.1007/s12549-017-0306-z ORIGINAL PAPER A palaeoenvironmental reconstruction of the Middle Jurassic of Sardinia (Italy) based on integrated palaeobotanical, palynological and lithofacies data assessment Luca Giacomo Costamagna1 & Evelyn Kustatscher2,3 & Giovanni Giuseppe Scanu1 & Myriam Del Rio1 & Paola Pittau1 & Johanna H. A. van Konijnenburg-van Cittert4,5 Received: 15 May 2017 /Accepted: 19 September 2017 # The Author(s) 2017. This article is an open access publication Abstract During the Jurassic, Sardinia was close to con- diverse landscape with a variety of habitats. Collection- tinental Europe. Emerged lands started from a single is- and literature-based palaeobotanical, palynological and land forming in time a progressively sinking archipelago. lithofacies studies were carried out on the Genna Selole This complex palaeogeographic situation gave origin to a Formation for palaeoenvironmental interpretations. They evidence a generally warm and humid climate, affected occasionally by drier periods. Several distinct ecosystems can be discerned in this climate, including alluvial fans This article is a contribution to the special issue BJurassic biodiversity and with braided streams (Laconi-Gadoni lithofacies), paralic ^ terrestrial environments . swamps and coasts (Nurri-Escalaplano lithofacies), and lagoons and shallow marine environments (Ussassai- * Evelyn Kustatscher [email protected] Perdasdefogu lithofacies). The non-marine environments were covered by extensive lowland and a reduced coastal Luca Giacomo Costamagna and tidally influenced environment. Both the river and the [email protected] upland/hinterland environments are of limited impact for Giovanni Giuseppe Scanu the reconstruction. The difference between the composi- [email protected] tion of the palynological and palaeobotanical associations evidence the discrepancies obtained using only one of those Myriam Del Rio [email protected] proxies. -

Etude Sur L'origine Et L'évolution Des Variations Florales Chez Delphinium L. (Ranunculaceae) À Travers La Morphologie, L'anatomie Et La Tératologie
Etude sur l'origine et l'évolution des variations florales chez Delphinium L. (Ranunculaceae) à travers la morphologie, l'anatomie et la tératologie : 2019SACLS126 : NNT Thèse de doctorat de l'Université Paris-Saclay préparée à l'Université Paris-Sud ED n°567 : Sciences du végétal : du gène à l'écosystème (SDV) Spécialité de doctorat : Biologie Thèse présentée et soutenue à Paris, le 29/05/2019, par Felipe Espinosa Moreno Composition du Jury : Bernard Riera Chargé de Recherche, CNRS (MECADEV) Rapporteur Julien Bachelier Professeur, Freie Universität Berlin (DCPS) Rapporteur Catherine Damerval Directrice de Recherche, CNRS (Génétique Quantitative et Evolution Le Moulon) Présidente Dario De Franceschi Maître de Conférences, Muséum national d'Histoire naturelle (CR2P) Examinateur Sophie Nadot Professeure, Université Paris-Sud (ESE) Directrice de thèse Florian Jabbour Maître de conférences, Muséum national d'Histoire naturelle (ISYEB) Invité Etude sur l'origine et l'évolution des variations florales chez Delphinium L. (Ranunculaceae) à travers la morphologie, l'anatomie et la tératologie Remerciements Ce manuscrit présente le travail de doctorat que j'ai réalisé entre les années 2016 et 2019 au sein de l'Ecole doctorale Sciences du végétale: du gène à l'écosystème, à l'Université Paris-Saclay Paris-Sud et au Muséum national d'Histoire naturelle de Paris. Même si sa réalisation a impliqué un investissement personnel énorme, celui-ci a eu tout son sens uniquement et grâce à l'encadrement, le soutien et l'accompagnement de nombreuses personnes que je remercie de la façon la plus sincère. Je remercie très spécialement Florian Jabbour et Sophie Nadot, mes directeurs de thèse. -

JUDD W.S. Et. Al. (2002) Plant Systematics: a Phylogenetic Approach. Chapter 7. an Overview of Green
UNCORRECTED PAGE PROOFS An Overview of Green Plant Phylogeny he word plant is commonly used to refer to any auto- trophic eukaryotic organism capable of converting light energy into chemical energy via the process of photosynthe- sis. More specifically, these organisms produce carbohydrates from carbon dioxide and water in the presence of chlorophyll inside of organelles called chloroplasts. Sometimes the term plant is extended to include autotrophic prokaryotic forms, especially the (eu)bacterial lineage known as the cyanobacteria (or blue- green algae). Many traditional botany textbooks even include the fungi, which differ dramatically in being heterotrophic eukaryotic organisms that enzymatically break down living or dead organic material and then absorb the simpler products. Fungi appear to be more closely related to animals, another lineage of heterotrophs characterized by eating other organisms and digesting them inter- nally. In this chapter we first briefly discuss the origin and evolution of several separately evolved plant lineages, both to acquaint you with these important branches of the tree of life and to help put the green plant lineage in broad phylogenetic perspective. We then focus attention on the evolution of green plants, emphasizing sev- eral critical transitions. Specifically, we concentrate on the origins of land plants (embryophytes), of vascular plants (tracheophytes), of 1 UNCORRECTED PAGE PROOFS 2 CHAPTER SEVEN seed plants (spermatophytes), and of flowering plants dons.” In some cases it is possible to abandon such (angiosperms). names entirely, but in others it is tempting to retain Although knowledge of fossil plants is critical to a them, either as common names for certain forms of orga- deep understanding of each of these shifts and some key nization (e.g., the “bryophytic” life cycle), or to refer to a fossils are mentioned, much of our discussion focuses on clade (e.g., applying “gymnosperms” to a hypothesized extant groups. -

From the Yixian Formation in Northeastern China
An early infructescence Hyrcantha decussata (comb. nov.) from the Yixian Formation in northeastern China David L. Dilcher*†, Ge Sun†‡, Qiang Ji§, and Hongqi Li¶ *Florida Museum of Natural History, University of Florida, Gainesville, FL 32611; ‡Research Center of Paleontology, Jilin University, Changchun 130026, China; §Geological Institute of Chinese Academy of Geosciences, Beijing 100037, China; and ¶Department of Biology, Frostburg State University, Frostburg, MD 21532 Contributed by David L. Dilcher, April 16, 2007 (sent for review November 10, 2006) The continuing study of early angiosperms from the Yixian For- about half their length. Each carpel containing 10–16 anatrop- mation (Ϸ125 Ma) of northeastern China has yielded a second early ous ovules/seeds borne along an adaxial linear placentae. angiosperm genus. This report is a detailed account of this early flowering plant and recognizes earlier reports of similar fossils H. decussata (Leng et Friis) Dilcher, Sun, Ji et Li comb. nov. from Russia and China. Entire plants, including roots, stems, and Synonymy. S. decussatus Leng et Friis, 2003, 2006. branches terminating in fruits are presented and reconstructed. Emended Description. Plant erect, 20–25 cm tall, with predomi- Evidence for a possible aquatic nature of this plant is presented. nately alternate branching of 30–45°, rarely ternate branching The relationship of Hyrcantha (‘‘Sinocarpus’’) to the eudicots is three to four times (Fig. 1A). Main axis 2.2–2.5 mm wide and discussed. The presence of this second early angiosperm genus, lightly striated alternate branches 1–1.2 mm wide. Lower now known as a whole plant, is important in the discussion of its branches with dilated nodes ensheathed by a thin ocrea (Fig. -

Palynostratigraphy and Palaeoenvironment of the Middle Jurassic Sortehat Formation (Neill Klinter Group), Jameson Land, East Greenland
Palynostratigraphy and palaeoenvironment of the Middle Jurassic Sortehat Formation (Neill Klinter Group), Jameson Land, East Greenland Eva B. Koppelhus and Carina F.Hansen The grey–black mudstones of the Sortehat Formation form part of the Middle Jurassic fill of the Jameson Land Basin in East Greenland. The formation is exposed in the southernmost part of the north–south-trending, Mesozoic rift system in East Greenland that was part of the epeiric sea- way between East Greenland and Norway. Sedimentological observations of the Sortehat Formation indicate deposition in an offshore marine setting that was typically low energy and periodically oxygen-deficient but was influenced by storm currents on occasion. Detailed palynological stud- ies of the Sortehat Formation have resulted in the definition of three palynological assemblage zones recognised at four localities, namely Enhjørningen Dal and Pelion (north Jameson Land), the type section at Sortehat (central Jameson Land) and Albuen at Neill Klinter along Hurry Inlet (south-east Jameson Land). In stratigraphic order, these zones are termed the Botryococcus Assemblage Zone, the Nannoceratopsis gracilis – Nannoceratopsis senex Assemblage Zone, and the Sentusidinium pelionense Assemblage Zone. They are recognised on the basis of the iden- tification of approximately 110 species of palynomorphs, including 45 species of spores, 30 of pollen, 22 of dinoflagellate cysts, 10 acritarch species, two species of algae, and some fungal spores. An Aalenian – ?Early Bajocian age is suggested for the Sortehat Formation on the basis of the palynoflora. Interpretation of the palynomorph assemblages suggests that the formation accumulated in a shallow, brackish marine environment. A significant terrestrial input, including the freshwater green alga Botryococcus, is recorded in the lower part of the formation and interpreted as an allochtho- nous accumulation in an offshore marine environment related to transgression of a low-lying coastal plain. -

The Evolutionary History of Flowering Plants
Journal & Proceedings of the Royal Society of New South Wales, vol. 149, parts 1 & 2, 2016, pp. 65–82. ISSN 0035-9173/16/010065–18 The evolutionary history of flowering plants Charles S.P. Foster1 1 School of Life and Environmental Sciences, University of Sydney, New South Wales 2006, Australia This paper was an RSNSW Scholarship Winner in 2015 Email: [email protected] Abstract In terms of species richness and important ecological roles, there are few biological groups that rival the success of flowering plants (Angiospermae). Angiosperm evolution has long been a topic of interest, with many attempts to clarify their phylogenetic relationships and timescale of evolution. However, despite this attention there remain many unsolved questions surrounding how and when flowers first appeared, and much of the angiosperm diversity remains to be quantified. Here, I review the evolutionary history of angiosperms, and how our understanding of this has changed over time. I begin by summarising the incredible morphological and genetic diversity of flowering plants, and the ways in which this can be studied using phylogenetic inference. I continue by discussing both the relationships between angiosperms and the other major lineages of seed plants, and the relationships between the main groups within angiosperms. In both cases, I outline how our knowledge has changed over time based on factors such as the different conclusions drawn from morphological and genetic data. I then discuss attempts to estimate the timescale of angiosperm evolution and the difficulties of doing so, including the apparent conflict between ages derived from fossil and molecular evidence. Finally, I propose future directions for angiosperm research to help clarify the evolutionary history of one of the most important groups of organisms on the planet. -

Ecological Sorting of Vascular Plant Classes During the Paleozoic Evolutionary Radiation
i1 Ecological Sorting of Vascular Plant Classes During the Paleozoic Evolutionary Radiation William A. DiMichele, William E. Stein, and Richard M. Bateman DiMichele, W.A., Stein, W.E., and Bateman, R.M. 2001. Ecological sorting of vascular plant classes during the Paleozoic evolutionary radiation. In: W.D. Allmon and D.J. Bottjer, eds. Evolutionary Paleoecology: The Ecological Context of Macroevolutionary Change. Columbia University Press, New York. pp. 285-335 THE DISTINCTIVE BODY PLANS of vascular plants (lycopsids, ferns, sphenopsids, seed plants), corresponding roughly to traditional Linnean classes, originated in a radiation that began in the late Middle Devonian and ended in the Early Carboniferous. This relatively brief radiation followed a long period in the Silurian and Early Devonian during wrhich morphological complexity accrued slowly and preceded evolutionary diversifications con- fined within major body-plan themes during the Carboniferous. During the Middle Devonian-Early Carboniferous morphological radiation, the major class-level clades also became differentiated ecologically: Lycopsids were cen- tered in wetlands, seed plants in terra firma environments, sphenopsids in aggradational habitats, and ferns in disturbed environments. The strong con- gruence of phylogenetic pattern, morphological differentiation, and clade- level ecological distributions characterizes plant ecological and evolutionary dynamics throughout much of the late Paleozoic. In this study, we explore the phylogenetic relationships and realized ecomorphospace of reconstructed whole plants (or composite whole plants), representing each of the major body-plan clades, and examine the degree of overlap of these patterns with each other and with patterns of environmental distribution. We conclude that 285 286 EVOLUTIONARY PALEOECOLOGY ecological incumbency was a major factor circumscribing and channeling the course of early diversification events: events that profoundly affected the structure and composition of modern plant communities. -

Fundamentals of Palaeobotany Fundamentals of Palaeobotany
Fundamentals of Palaeobotany Fundamentals of Palaeobotany cuGU .叮 v FimditLU'φL-EjAA ρummmm 吋 eαymGfr 伊拉ddd仇側向iep M d、 況 O C O W Illustrations by the author uc削 ∞叩N Nn凹創 刊,叫MH h 咀 可 白 a aEE-- EEA First published in 1987 by Chapman αndHallLtd 11 New Fetter Lane, London EC4P 4EE Published in the USA by Chα~pman and H all 29 West 35th Street: New Yo地 NY 10001 。 1987 S. V. M秒len Softcover reprint of the hardcover 1st edition 1987 ISBN-13: 978-94-010-7916-7 e-ISBN-13: 978-94-009-3151-0 DO1: 10.1007/978-94-009-3151-0 All rights reserved. No part of this book may be reprinted, or reproduced or utilized in any form or by any electronic, mechanical or other means, now known or hereafter invented, including photocopying and recording, or in any information storage and retrieval system, without permission in writing from the publisher. British Library Cataloguing in Publication Data Mey凹, Sergei V. Fundamentals of palaeobotany. 1. Palaeobotany I. Title 11. Osnovy paleobotaniki. English 561 QE905 Library 01 Congress Catα loging in Publication Data Mey凹, Sergei Viktorovich. Fundamentals of palaeobotany. Bibliography: p. Includes index. 1. Paleobotany. I. Title. QE904.AIM45 561 8ι13000 Contents Foreword page xi Introduction xvii Acknowledgements xx Abbreviations xxi 1. Preservation 抄'pes αnd techniques of study of fossil plants 1 2. Principles of typology and of nomenclature of fossil plants 5 Parataxa and eutaxa S Taxa and characters 8 Peculiarity of the taxonomy and nomenclature of fossil plants 11 The binary (dual) system of fossil plants 12 The reasons for the inflation of generic na,mes 13 The species problem in palaeobotany lS The polytypic concept of the species 17 Assemblage-genera and assemblage-species 17 The cladistic methods 18 3. -

UC Davis UC Davis Previously Published Works
UC Davis UC Davis Previously Published Works Title Integrating early Cretaceous fossils into the phylogeny of living angiosperms: Anita lines and relatives of Chloranthaceae Permalink https://escholarship.org/uc/item/3bj1s569 Journal International Journal of Plant Sciences, 175(5) ISSN 1058-5893 Authors Doyle, JA Endress, PK Publication Date 2014 DOI 10.1086/675935 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Int. J. Plant Sci. 175(5):555–600. 2014. ᭧ 2014 by The University of Chicago. All rights reserved. 1058-5893/2014/17505-0006$15.00 DOI: 10.1086/675935 INTEGRATING EARLY CRETACEOUS FOSSILS INTO THE PHYLOGENY OF LIVING ANGIOSPERMS: ANITA LINES AND RELATIVES OF CHLORANTHACEAE James A. Doyle1,* and Peter K. Endress† *Department of Evolution and Ecology, University of California, Davis, California 95616, USA; and †Institute of Systematic Botany, University of Zurich, 8008 Zurich, Switzerland Editor: Patrick S. Herendeen Premise of research. Discoveries of fossil flowers in Cretaceous rocks offer improved evidence for rela- tionships with living clades, but for more secure inferences formal phylogenetic analyses are desirable. We extend previous analyses of magnoliids, monocots, and basal eudicots to Aptian, Albian, and Cenomanian fossils related to the basal “ANITA” lines and Chloranthaceae. Methodology. We performed parsimony analyses of a morphological data set of Recent angiosperms and published fossils, with the arrangement of Recent taxa constrained to backbone trees based primarily on molecular data. Pivotal results. Not only Monetianthus (as previously inferred) but also Carpestella is nested within Nymphaeaceae, while Pluricarpellatia may be a stem relative of Cabombaceae or Nymphaeaceae. Anacostia (with Similipollis pollen) is nested within Austrobaileyales. -
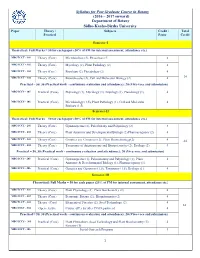
Syllabus for Post Graduate Course in Botany (2016 – 2017 Onward)
Syllabus for Post Graduate Course in Botany (2016 – 2017 onward) Department of Botany Sidho-Kanho-Birsha University Paper Theory / Subjects Credit / Total Practical Paper Credit Semester-I Theoretical: Full Marks = 50 for each paper (20% of FM for internal assessment, attendance etc.) MBOTCCT - 101 Theory (Core) Microbiology (2), Phycology (2) 4 MBOTCCT - 102 Theory (Core) Mycology (2), Plant Pathology (2) 4 MBOTCCT - 103 Theory (Core) Bryology (2), Pteridology (2) 4 MBOTCCT - 104 Theory (Core) Biomolecules (2), Cell and Molecular Biology (2) 4 24 Practical = 50, 30 (Practical work - continuous evaluation and attendance); 20 (Viva-voce and submission) MBOTCCS - 105 Practical (Core) Phycology (1), Mycology (1), Bryology (1), Pteridology (1). 4 MBOTCCS - 106 Practical (Core) Microbiology (1.5), Plant Pathology (1), Cell and Molecular 4 Biology (1.5). Semester-II Theoretical: Full Marks = 50 for each paper (20% of FM for internal assessment, attendance etc.) MBOTCCT - 201 Theory (Core) Gymnosperms (2), Paleobotany and Palynology (2) 4 MBOTCCT - 202 Theory (Core) Plant Anatomy and Developmental Biology (2) Pharmacognosy (2) 4 MBOTCCT - 203 Theory (Core) Genetics and Genomics (2), Plant Biotechnology(2) 4 24 MBOTCCT - 204 Theory (Core) Taxonomy of Angiosperms and Biosystematics (2), Ecology (2) 4 Practical = 50, 30 (Practical work - continuous evaluation and attendance); 20 (Viva-voce and submission) MBOTCCS - 205 Practical (Core) Gymnosperms (1), Palaeobotany and Palynology (1), Plant 4 Anatomy & Developmental Biology (1), Pharmacognosy (1). MBOTCCS - 206 Practical (Core) Genetics and Genomics (1.5), Taxonomy (1.5), Ecology (1). 4 Semester-III Theoretical: Full Marks = 50 for each paper (20% of FM for internal assessment, attendance etc.) MBOTCCT - 301 Theory (Core) Plant Physiology (2), Plant Biochemistry (2) 4 MBOTCCT - 302 Theory (Core) Economic Botany (2), Bioinformatics (2) 4 MBOTCCT - 303 Theory (Core) Elements of Forestry (2), Seed Technology (2). -

Cretaceous Blog
A Quick Look at the Cretaceous World David Lillis – 13 May 2020 e-mail: [email protected] The Extent of the Cretaceous The Cretaceous Period began about 144 million years ago and terminated with the well- known asteroid impact some 66 million years ago. Geologists subdivide it into 12 stages, each defined by particular rock formations, fossils and sediments at a specific locality called the type area. Several of these type areas are located in France (e.g. Cognac, France, is the type area for the Coniacian Stage). These stages lasted for several million years each, and much geological, climatic and evolutionary change took place within each of them. Figure 1 gives the time frames of the twelve Cretaceous stages. Figure 1: The Cretaceous and its twelve stages The Cretaceous stages vary in duration but average somewhat less than seven million years. The Aptian stage has the greatest duration, at about 12 million years; while the shortest stage is the Santonian, at below three million years. The final stage is known as the Maastrichtian (approximately 6.1 million years in duration), the stage that ended with the famous asteroid impact and mass extinction of dinosaurs and other life forms. Significant rock formations or the earliest appearance of particular organisms define both the lower and upper boundaries of these stages. For example, one marker for the base of the Maastrichtrian is the earliest occurrence of a marine mollusc, the ammonite Pachydiscus fresvillensis. 1 Figure 2 shows a fossilised Pachydiscus fresvillensis from Madagascar, dated at about 69 million years. Figure 2: A Pachydiscus fresvillensis fossil, about 69 million years old The Cretaceous lies within the Mesozoic Era, which we can think of as the age of dinosaurs.