Unconformity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Stratigraphic Framework of Cambrian and Ordovician Rocks in The
Stratigraphic Framework of Cambrian and Ordovician Rocks in the Central Appalachian Basin from Medina County, Ohio, through Southwestern and South-Central Pennsylvania to Hampshire County, West Virginia U.S. GEOLOGICAL SURVEY BULLETIN 1839-K Chapter K Stratigraphic Framework of Cambrian and Ordovician Rocks in the Central Appalachian Basin from Medina County, Ohio, through Southwestern and South-Central Pennsylvania to Hampshire County, West Virginia By ROBERT T. RYDER, ANITA G. HARRIS, and JOHN E. REPETSKI Stratigraphic framework of the Cambrian and Ordovician sequence in part of the central Appalachian basin and the structure of underlying block-faulted basement rocks U.S. GEOLOGICAL SURVEY BULLETIN 1839 EVOLUTION OF SEDIMENTARY BASINS-APPALACHIAN BASIN U.S. DEPARTMENT OF THE INTERIOR MANUEL LUJAN, Jr., Secretary U.S. GEOLOGICAL SURVEY Dallas L. Peck, Director Any use of trade, product, or firm names in this publication is for descriptive purposes only and does not imply endorsement by the U.S. Government UNITED STATES GOVERNMENT PRINTING OFFICE: 1992 For sale by Book and Open-File Report Sales U.S. Geological Survey Federal Center, Box 25286 Denver, CO 80225 Library of Congress Cataloging in Publication Data Ryder, Robert T. Stratigraphic framework of Cambrian and Ordovician rocks in the central Appalachian Basin from Medina County, Ohio, through southwestern and south-central Pennsylvania to Hampshire County, West Virginia / by Robert T. Ryder, Anita C. Harris, and John E. Repetski. p. cm. (Evolution of sedimentary basins Appalachian Basin ; ch. K) (U.S. Geological Survey bulletin ; 1839-K) Includes bibliographical references. Supt. of Docs, no.: I 19.3:1839-K 1. Geology, Stratigraphic Cambrian. -

Stratigraphical Framework for the Devonian (Old Red Sandstone) Rocks of Scotland South of a Line from Fort William to Aberdeen
Stratigraphical framework for the Devonian (Old Red Sandstone) rocks of Scotland south of a line from Fort William to Aberdeen Research Report RR/01/04 NAVIGATION HOW TO NAVIGATE THIS DOCUMENT ❑ The general pagination is designed for hard copy use and does not correspond to PDF thumbnail pagination. ❑ The main elements of the table of contents are bookmarked enabling direct links to be followed to the principal section headings and sub-headings, figures, plates and tables irrespective of which part of the document the user is viewing. ❑ In addition, the report contains links: ✤ from the principal section and sub-section headings back to the contents page, ✤ from each reference to a figure, plate or table directly to the corresponding figure, plate or table, ✤ from each figure, plate or table caption to the first place that figure, plate or table is mentioned in the text and ✤ from each page number back to the contents page. Return to contents page NATURAL ENVIRONMENT RESEARCH COUNCIL BRITISH GEOLOGICAL SURVEY Research Report RR/01/04 Stratigraphical framework for the Devonian (Old Red Sandstone) rocks of Scotland south of a line from Fort William to Aberdeen Michael A E Browne, Richard A Smith and Andrew M Aitken Contributors: Hugh F Barron, Steve Carroll and Mark T Dean Cover illustration Basal contact of the lowest lava flow of the Crawton Volcanic Formation overlying the Whitehouse Conglomerate Formation, Trollochy, Kincardineshire. BGS Photograph D2459. The National Grid and other Ordnance Survey data are used with the permission of the Controller of Her Majesty’s Stationery Office. Ordnance Survey licence number GD 272191/2002. -

A Silurian(?) Unconformity at Flanders Bay, Maine
Maine Geological Survey Studies in Maine Geology: Volume I 1988 A Silurian (?) Unconformity at Manders Bay, Maine Richard A. Gilman Gary G. Lash Department of Geosciences State University College at Fredonia (SUNY) Fredonia, New York 14063 ABSTRACT Reexamination of exposures of conglomerate and greenstone at the head of Flanders Bay has resulted in the recognition of an unconformity believed similar to that exposed on Spectacle Island on the east side of Frenchman Bay. It is proposed that the conglomerate is part of the Bar Harbor Formation of Siluro-Devonian age; the underlying greenstone is probably of Silurian age. The rocks above the unconformity consist of pebbly siltstone and polymict, matrix-supported conglomerate in which clasts are predominantly of felsic volcanic rocks and quartz. A depositional model is proposed in which gravels derived from a volcanic source terrane, and transported in a high energy tluvial system, were mixed with basin-margin muds to produce debris tlows. Chemical analyses of the underlying greenstone show it to be andesitic, but to have substantial differences in composition from Silurian and Lower Devonian basalts and basaltic andesites from the Machias-Eastport area. INTRODUCTION In his study of the stratigraphy of the Bar Harbor Formation 68° 15' 68° 00' in the Frenchman Bay area, Maine, Metzger (1979) briefly men 44° 30·~-~-~-~~-----------~ 2) tions exposures of pebble conglomerates that contain clasts of volcanic rocks and quartz at several isolated outcrops at the head of Flanders Bay, in the vicinity of Jones Cove (Fig. l). The ~N conglomerates are associated with a weakly foliated greenstone. He concluded that the conglomerates are intraformational with \ones Cove in a sequence of greenstones rather than belonging to the con Frenchman Bay glomerates of the Bar Harbor Formation that he describes from Spectacle, Turtle, Flat and Heron Islands on the east side of \) SCHOODIC Frenchman Bay (see also Gates, in press). -

EPGS Guidebook
THE EL PAS0 GEOLOGICAL SOCIETY GUIDEBOOK FOURTH ANNUAL FIELD TRIP CENOZOIC STRATIGRAPHY Of THE RIO GRANDE VALLEY AREA DORA ANA COUNTY NEW MEXICO MARCH 14, 1970 CENOZOIC STRATIGRAPHY OF THE RIO GRANDE VALLEY AREA DQk ANA COUNTY, NEW MEXICO John W. Hawley - Editor and Cmpi ler GUIDEBOOK FOURTH ANNUAL FIELD TRIP of the EL PAS0 GEOLOGICAL SOCIETY March 14, 1970 Compiled in Cooperati on with: Department of Geological Sciences, University of Texas at El Paso Earth Sciences and Astronomy Department, New Mexi co State University Soi 1 Survey Investigations, SCS, USDA, University Park, New Mexico New Mexico State Bureau of Mines and Mineral Resources, Socorro, New Mexico EL PAS0 GEOLOGICAL SOCIETY OFFICERS Charles J. Crowley Presi dent El Paso Natural Gas C. Tom Hollenshead Vice President El Paso Natural Gas Carl Cotton Secretary El Paso Indpt. School Dist. Thomas F. Cliett Treasurer El Paso Water Utilities Wi11 iam N. McAnul ty Counci lor Dept. Geol. Sci., UTEP Robert D. Habbit Councilor El Paso Natural Gas FIELD TRIP COMMITTEES Guidebook John W. Hawley Edi tor and compi 1er Soi 1 Survey Invest., SCS Jerry M. Hoffer Contributor and editing Dept. Geol. Sci., UTEP William R. Seager Contributor and editing Earth Sci. Dept. NMSU Frank E. Kottlowski Contributor and editing N. M. Bur. Mines & Min. Res. Earl M.P. Lovejoy Contributor and editing Dept. Geol. Sci., UTEP William S. Strain Contributor and editing Dept. Geol. Sci., UTEP Paul a Blackshear Typing Dept. Geol . Sci ., UTEP Robert Sepul veda Drafting Dept. Geol . Sci ., UTEP Caravan Earl M. P. Lovejoy Pub1 icity and Regi stration Charles J. -

The Old Red Sandstone of Britain and Ireland – a Review
The Old Red Sandstone of Britain and Ireland – a review RS Kendall British Geological Survey - Cardiff University, Main Building, Park Place, Cardiff. CF10 3AT. [email protected] Abstract The Old Red Sandstone (ORS) is an informal term which is given to continental, predominantly siliclastic, strata of late Silurian to early Carboniferous age which were deposited across the continent of Laurussia at sub-tropic to tropical latitudes. The coincidental development of land plants had a major impact on the atmosphere and global climate by lowering atmospheric carbon dioxide levels, which profoundly affected the style of alluvial sedimentation during this interval, by stabilising flood plains and facilitating the development of soils. The ORS also provides examples of syn- to post- orogenic deposition related to the Caledonian Orogeny, which was affected by synchronous tectonism and volcanism. The influence of Variscan tectonics on basin deformation and tectonism are also evident in the ORS sequence. In October 2014, a symposium was held, organised by the South Wales Geologists’ Association, entitled The Old Red Sandstone: is it Old, is it Red and is it all Sandstone? The event consisted of talks and posters on topics associated with the Old Red Sandstone deposits, principally of Wales and the Welsh Borders and the Scottish Borders in the UK, and included a series of field trips. Seven of the speakers have contributed manuscripts which are presented in this volume. These include papers discussing fossil fish and plant assemblages, the Fforest Fawr Geopark, Old Red Sandstone building stones, and soft sediment deformation. A brief report on the event and acknowledgements is also included. -

Stratigraphic Cross Sections—Why Study Old Rocks?
From IRIS’ collection: Animations of Geologic Processes www.iris.edu/educate/animations Stratigraphic Cross Sections—Why study old rocks? The earthquake potential of an area can be DEFINITIONS determined by studying the relationships between Stratigraphy— The branch of geology that rock strata, both locally and regionally. Faults and folds studies rock layers (strata) & layering record a probable earthquake history, so by studying (stratification). It is primarily used in the the age of the rocks we can gain an understanding study of sedimentary & layered volcanic about future earthquakes (applying “the past is a key to rocks. the future”). This is done by doing field work, studying drill-hole samples, and interpreting seismic reflection Cross section—A view formed by a plane data. cutting through an object to reveal a profile (a vertical section of the Earth’s crust showing the different horizons or This animation, has both stratification and structure. layers). No strata are overturned, thus the following laws apply: Stratigraphic section—Also called The law of superposition simply states that for non- “stratigraphic cross section”, is a deformed geologic strata, “sedimentary layers are cutaway view, usually with the vertical deposited in a time sequence, with the oldest on the scale exaggerated with relation to the bottom and the youngest on the top”. horizontal scale, that is designed to The principle of original horizontality states that rock show the thicknesses & relationships layers form in the horizontal position, and any between successions of rock types. deviations from this horizontal position are due to Structural geology—Used to uncover the rocks being disturbed later (the exception being information about faults & folds in rocks. -

Basin Evolution and Shale Tectonics on an Obliquely Convergent Margin: the Bahia Basin, Offshore Colombian Caribbean
Basin evolution and shale tectonics on an obliquely convergent margin: the Bahia Basin, offshore Colombian Caribbean Pedro A. Galindo1* and Lidia Lonergan1 1 Department of Earth Science and Engineering, Imperial College London Corresponding author; Pedro Galindo ([email protected]) * Current address: Ecopetrol S.A., Bogotá, Colombia Key Points: In response to oblique convergence, strike-slip deformation occurs at the rear of the South Caribbean Deformed Belt and controls the evolution of the Bahia Basin Strike-slip deformation and sediment loading triggered the movement of overpressured shale, creating mud-withdrawal minibasins Strain-partitioning led to the break-up and lateral displacement of accretionary prisms formed along the margin, opening the space for depocenters This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process which may lead to differences between this version and the Version of Record. Please cite this article as doi: 10.1029/2019TC005787 ©2019 American Geophysical Union. All rights reserved. Abstract Oblique convergent margins accumulate strike-slip deformation that controls basin formation and evolution. The Bahia Basin is located offshore, proximal to major strike-slip fault systems that affect northern Colombia. It lies behind the toe of the modern accretionary prism, where the Caribbean Plate is being subducted obliquely beneath South America. This is the first attempt using 3D seismic reflection data to interpret a complex strike-slip basin at the western end of the southern margin of the Caribbean Plate. Detailed 2D and 3D seismic mapping of regional unconformities and faults is used to describe the structural geometry, timing and evolution of extensional and strike-slip faults which controlled the formation of the basin. -

Geologic Dating
DATE DUE: NAME: INSTRUCTOR: TERRY J. BOROUGHS Geology 305 SECTION: GEOLOGICAL TIME / DATING TECHNIQUES Instructions: Read each question carefully before selecting the BEST answer. Provide specific and detailed answers to essay questions. Use GEOLOGIC VOCABULARY where APPLICABLE! USE AN 882 SCANTRON TO RECORD YOUR ANSWERS FOR THE FOLLOWING MULTIPLE CHOICE AND T/F QUESTIONS. TURN IN YOUR SCANTRON AND ANSWER SHEET ONLY! Multiple Choice: 1. About 88 percent of geologic time is represented by the time span often called the: a. Paleozoic. b. Stone Age. c. Precambrian. d. Cretaceous. e. Ordovician. 2. The statement "The present is the key to the past" relates to a. Principle of Catastrophism c. Principle of Uniformitarianism b. Aristotle's hypothesis d. Tarbuck's theory e. none of these 3. Most ancient rocks on the surface of the Earth have been recycled by plate tectonics, but there are ancient rocks on Earth that have been discovered to be approximately years old. a. 4 thousand b. 4 million c. 4 billion d. 4 trillion 4. Which one of the following represents the longest expanse of time? a. Mesozoic b. Cenozoic c. Precambrian d. Paleozoic 5. The forces and processes that can be observed shaping our present day landscapes have also operated in the geologic past. The preceding statement refers to: a. Principle of Uniformitarianism d. Principle of Catastrophism b. Aristotle's hypothesis e. Principle of Uniformity c. Answers a. and e. f. Answers b. and d. 6. The principle of fossil succession states that: a. the same sequence of life forms repeats over and over b. -

Colombian Sedimentary Basins: Nomenclature, Boundaries and Petroleum Geology, a New Proposal
ANH AGENCIA NACIONAL DE HIDROCARBUROS By: Darío Barrero1 Andrés Pardo2, 3 Carlos A. Vargas2, 4 Juan F. Martínez1 1B & M Exploration Ltda, Bogotá 2Agencia Nacional de Hidrocarburos (ANH) 3Universidad de Caldas, Departamento de Ciencias Geológicas, Manizales 4Universidad Nacional de Colombia, Departamento de Geociencias, Bogotá Colombian Sedimentary Basins: Nomenclature, Boundaries and Petroleum Geology, a New Proposal 2 President of the Republic of Colombia ÁLVARO URIBE VÉLEZ Minister of Mines and Energy HERNÁN MARTÍNEZ TORRES General Director ANH JOSÉ ARMANDO ZAMORA REYES Technical Sub-director ROGELIO TORO LONDOÑO Chief of Geologists CARLOS A. VARGAS JIMÉNEZ For information, please contact: AGENCIA NACIONAL DE HIDROCARBUROS – A.N.H.- Calle 99 No. 9 A - 54 (piso 14) - Bogotá, Colombia Teléfono: (571) 593 17 17 - Fax: (571) 593 17 18 www.anh.gov.co [email protected] Línea Gratuita Nacional 018000 953 000 3 ISBN: 978-958-98237-0-5 Edited and compiled by: ANH and B&M Exploration Ltda. Copyright © 2007 Printed in Bogotá-Colombia • 2.007 The editor makes no representation, Express or implied, with regard to the accuracy of the information contained in this document and cannot accept any legal responsibility or liability for any errors or omissions that may be made. Table of Contents Table of Contents THE NATIONAL HIDROCARBONS AGENCY - A.N.H. - MISSION 8 VISION 8 FUNCTIONS 9 GENERAL INFORMATION ABOUT COLOMBIA 11 Geographic Location 11 Climate 11 Population and Language 11 Type of Government 11 2006 Economic Indicators 11 Main Imports 11 Main Exports 11 1. SEDIMENTARY BASINS OF COLOMBIA: 5 GEOLOGICAL FRAMEWORK 14 2. REVIEW OF NOMENCLATURE AND BOUNDARIES OF COLOMBIAN BASINS 22 2.1 Brief Historical Overview 25 2.2 Colombian Basin Nomenclature and Boundaries 27 2.3 Proposed Modifi cations to the Sedimentary Basin Map of Colombia 29 2.4 Proposed Boundaries 29 3. -
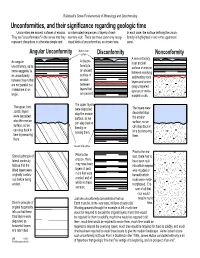
Unconformities, and Their Significance Regarding Geologic Time Uncormities Are Ancient Surfaces of Erosion
Railsback's Some Fundamentals of Mineralogy and Geochemistry Unconformities, and their significance regarding geologic time Uncormities are ancient surfaces of erosion. un-interrupted sequences of layers of sedi- In each case, the surface defining the uncon- They are "unconformable" in the sense that they mentary rock. There are three commonly recog- formity is highlighted in red in the uppermost represent disruptions in otherwise simple and nized kinds of unconformities, as shown here. panel. Modern land Angular Unconformity surface Disconformity Nonconformity A nonconformity A discon- An angular is an ancient formity is unconformity, as its surface of erosion an ancient name suggests, is between overlying surface of an unconformity sedimentary rock erosion between layers that layers and under- are not parallel but between lying unlayered layers that instead are at an igneous or meta- are parallel. angle. morphic rocks. The upper layers The upper, hori- were deposited The layers were zontal, layers atop the erosion deposited atop were deposited surface, so we the erosion atop the erosion can step back in surface, so we surface, so we time by re- can step back in can step back in moving them. time by removing time by removing them. them. Ancient land surface Prior to the ero- Steno's principle of Prior to the sion, there has to lateral continuity erosion, there have been rock tells us that the may have been into which magma tillted layers were layers of sedi- was intruded or originally continu- ment that were beneath which ous before being eroded, and of rocks were meta- eroded. which no trace morphosed. -

Chapter 19. Measuring Geological Time
Physical Geology, First University of Saskatchewan Edition is used under a CC BY-NC-SA 4.0 International License Read this book online at http://openpress.usask.ca/physicalgeology/ Chapter 19. Measuring Geological Time Adapted by Tim Prokopiuk and Karla Panchuk from Physical Geology by Steven Earle Figure 19.1 Arizona’s Grand Canyon is an icon for geological time; 1,450 million years are represented by this photo. The light-coloured layers of rocks at the top formed at ~ 250 Ma, and the dark ones at the bottom of the canyon at ~ 1,700 Ma. Source: Steven Earle (2015) CC BY 4.0 Learning Objectives After reading this chapter and answering the Review Questions at the end, you should be able to: • Apply basic geological principles to determine the relative ages of rocks. • Explain the difference between relative and absolute age-dating techniques. • Summarize the history of the geological time scale and the relationships between eons, eras, periods, and epochs. • Understand the importance and significance of unconformities. • Estimate the age of a rock based on the fossils that it contains. • Use isotopic data to estimate the absolute age of a rock. • Describe some applications and limitations of isotopic techniques for absolute geological dating. • Describe the techniques for dating geological materials using tree rings and magnetic data. • Explain why an understanding of geological time is critical to both geologists and the public in general. Geological Time Is Vast Time is the dimension that sets geology apart from most other sciences. Geological time is vast, and Earth has changed tremendously during this time. -

Carboniferous Petroleum Systems Beneath the Central North
2013 2010 2011 2012 Carboniferous petroleum systems Promote beneath the Central North Sea United Kingdom 2014 1. Abstract Interpretation of recent long-offset seismic data augmented by potential field modelling has revealed a Devono- Carboniferous basin architecture beneath the Central North Sea that exhibits many features in common with the contemporary basins of northern England and southern Scotland (Milton-Worssell et al. in press). Potential source rock intervals within Dinantian and Westphalian-age coal measures remote from the traditional Central Graben Jurassic source kitchen are confirmed by deep Central North Sea wells. The maturity of these potential source rocks is supported by the occurrence of prominent gas chimneys in the Mesozoic and Tertiary strata of southern Quadrant 29, up to 50 km south-west of the Jurassic source kitchen (Hay et al. 2005). 2. Middle Devonian to Lower Carboniferous basin architecture Seismic interpretation has provided indications that many of the basement controls that defined the architecture of the onshore UK Lower Carboniferous basins operated across much of the UK Central North Sea also (see section 5). In the south-east Central North Sea (Fig. 1), Mid North Sea High structural elements have been interpreted that are comparable with the Askrigg Block and Stainmore Trough. The up-thrust Auk-Flora Ridge intra-basinal basement block resembles the Pentland Hills inlier within the Midland Valley of Scotland and the Cross Fell inlier in northern England. SOUTHERN GRANITE-CORED MID-NORTH SEA HIGH CENTRAL NORTH BLOCK CARBONIFEROUS BASIN GRABEN SEA (cf. Askrigg Block) (cf. Stainmore Trough) u/c base Cret. top Hod Zechstein Gp.