Restricting Calcium Currents Is Required for Correct Fiber Type
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Spectrum of CLCN1 Mutations in Patients with Myotonia Congenita in Northern Scandinavia
European Journal of Human Genetics (2001) 9, 903 ± 909 ã 2001 Nature Publishing Group All rights reserved 1018-4813/01 $15.00 www.nature.com/ejhg ARTICLE Spectrum of CLCN1 mutations in patients with myotonia congenita in Northern Scandinavia Chen Sun*,1, Lisbeth Tranebjñrg*,1, Torberg Torbergsen2,GoÈsta Holmgren3 and Marijke Van Ghelue1,4 1Department of Medical Genetics, University Hospital of Tromsù, Tromsù, Norway; 2Department of Neurology, University Hospital of Tromsù, Tromsù, Norway; 3Department of Clinical Genetics, University Hospital of UmeaÊ, UmeaÊ,Sweden;4Department of Biochemistry, Section Molecular Biology, University of Tromsù, Tromsù, Norway Myotonia congenita is a non-dystrophic muscle disorder affecting the excitability of the skeletal muscle membrane. It can be inherited either as an autosomal dominant (Thomsen's myotonia) or an autosomal recessive (Becker's myotonia) trait. Both types are characterised by myotonia (muscle stiffness) and muscular hypertrophy, and are caused by mutations in the muscle chloride channel gene, CLCN1. At least 50 different CLCN1 mutations have been described worldwide, but in many studies only about half of the patients showed mutations in CLCN1. Limitations in the mutation detection methods and genetic heterogeneity might be explanations. In the current study, we sequenced the entire CLCN1 gene in 15 Northern Norwegian and three Northern Swedish MC families. Our data show a high prevalence of myotonia congenita in Northern Norway similar to Northern Finland, but with a much higher degree of mutation heterogeneity. In total, eight different mutations and three polymorphisms (T87T, D718D, and P727L) were detected. Three mutations (F287S, A331T, and 2284+5C4T) were novel while the others (IVS1+3A4T, 979G4A, F413C, A531V, and R894X) have been reported previously. -

Unnatural Verticilide Enantiomer Inhibits Type 2 Ryanodine Receptor-Mediated Calcium Leak and Is Antiarrhythmic
Unnatural verticilide enantiomer inhibits type 2 ryanodine receptor-mediated calcium leak and is antiarrhythmic Suzanne M. Batistea,1, Daniel J. Blackwellb,1, Kyungsoo Kimb,1, Dmytro O. Kryshtalb, Nieves Gomez-Hurtadob, Robyn T. Rebbeckc, Razvan L. Corneac, Jeffrey N. Johnstona,2, and Bjorn C. Knollmannb,2 aDepartment of Chemistry, Vanderbilt University, Nashville, TN 37235; bDepartment of Medicine, Vanderbilt University Medical Center, Nashville, TN 37232; and cDepartment of Biochemistry, Molecular Biology, and Biophysics, University of Minnesota, Minneapolis, MN 55455 Edited by Dale L. Boger, The Scripps Research Institute, La Jolla, CA, and approved January 15, 2019 (received for review September 27, 2018) Ca2+ leak via ryanodine receptor type 2 (RyR2) can cause poten- heart diseases associated with both atrial and ventricular arrhyth- tially fatal arrhythmias in a variety of heart diseases and has also mia (9). Mutations in RyR2 and its binding partners, which increase + been implicated in neurodegenerative and seizure disorders, mak- SR Ca2 leak, cause primary atrial and ventricular arrhythmia ing RyR2 an attractive therapeutic target for drug development. syndromes such as catecholaminergic polymorphic ventricular Here we synthesized and investigated the fungal natural product tachycardia (CPVT), providing strong evidence for the mechanistic and known insect RyR antagonist (−)-verticilide and several conge- contribution of RyR2 to arrhythmia risk in humans (10). Further ners to determine their activity against mammalian RyR2. Although support comes from gene-targeted mouse models of CPVT, where + the cyclooligomeric depsipeptide natural product (−)-verticilide had catecholamine-induced spontaneous Ca2 release from the SR no effect, its nonnatural enantiomer [ent-(+)-verticilide] signifi- via RyR2 generates potentially fatal cardiac arrhythmias (11, 12). -

Calcium-Induced Calcium Release in Smooth Muscle7 Loose Coupling
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by PubMed Central Calcium-induced Calcium Release in Smooth Muscle✪ Loose Coupling between the Action Potential and Calcium Release M.L. Collier, G. Ji, Y.-X. Wang, and M.I. Kotlikoff From the Department of Animal Biology, School of Veterinary Medicine, University of Pennsylvania, Philadelphia, Pennsylvania 19104-6046 abstract Calcium-induced calcium release (CICR) has been observed in cardiac myocytes as elementary cal- cium release events (calcium sparks) associated with the opening of L-type Ca2ϩ channels. In heart cells, a tight coupling between the gating of single L-type Ca2ϩ channels and ryanodine receptors (RYRs) underlies calcium re- lease. Here we demonstrate that L-type Ca2ϩ channels activate RYRs to produce CICR in smooth muscle cells in the form of Ca2ϩ sparks and propagated Ca2ϩ waves. However, unlike CICR in cardiac muscle, RYR channel open- ing is not tightly linked to the gating of L-type Ca2ϩ channels. L-type Ca2ϩ channels can open without triggering Ca2ϩ sparks and triggered Ca2ϩ sparks are often observed after channel closure. CICR is a function of the net flux of Ca2ϩ ions into the cytosol, rather than the single channel amplitude of L-type Ca2ϩ channels. Moreover, unlike CICR in striated muscle, calcium release is completely eliminated by cytosolic calcium buffering. Thus, L-type Ca2ϩ channels are loosely coupled to RYR through an increase in global [Ca2ϩ] due to an increase in the effective distance between L-type Ca2ϩ channels and RYR, resulting in an uncoupling of the obligate relationship that exists in striated muscle between the action potential and calcium release. -

Characterization of Cecal Smooth Muscle Contraction in Laying Hens
veterinary sciences Communication Characterization of Cecal Smooth Muscle Contraction in Laying Hens Katrin Röhm 1, Martin Diener 2 , Korinna Huber 1 and Jana Seifert 1,* 1 Institute of Animal Science, University of Hohenheim, 70593 Stuttgart, Germany; [email protected] (K.R.); [email protected] (K.H.) 2 Institute of Veterinary Physiology and Biochemistry, Justus-Liebig University, 35392 Gießen, Germany; [email protected] * Correspondence: [email protected] Abstract: The ceca play an important role in the physiology of the gastrointestinal tract in chickens. Nevertheless, there is a gap of knowledge regarding the functionality of the ceca in poultry, especially with respect to physiological cecal smooth muscle contraction. The aim of the current study is the ex vivo characterization of cecal smooth muscle contraction in laying hens. Muscle strips of circular cecal smooth muscle from eleven hens are prepared to investigate their contraction ex vivo. Contraction is detected using an isometric force transducer, determining its frequency, height and intensity. Spontaneous contraction of the chicken cecal smooth muscle and the influence of buffers (calcium-free buffer and potassium-enriched buffer) and drugs (carbachol, nitroprusside, isoprenaline and Verapamil) affecting smooth muscle contraction at different levels are characterized. A decrease in smooth muscle contraction is observed when a calcium-free buffer is used. Carbachol causes an increase in smooth muscle contraction, whereas atropine inhibits contraction. Nitroprusside, isoprenaline and Verapamil result in a depression of smooth muscle contraction. In conclusion, the present results confirm a similar contraction behavior of cecal smooth muscles in laying hens as Citation: Röhm, K.; Diener, M.; shown previously in other species. -

Microarchitecture of the Dyad
Cardiovascular Research (2013) 98, 169–176 SPOTLIGHT REVIEW doi:10.1093/cvr/cvt025 Microarchitecture of the dyad David R.L. Scriven, Parisa Asghari, and Edwin D.W. Moore* Department of Cellular and Physiological Sciences, Life Sciences Institute, University of British Columbia, 2350 Health Sciences Mall, Vancouver, BC, Canada V6T 1Z3 Received 12 December 2012; revised 2 February 2013; accepted 4 February 2013; online publish-ahead-of-print 11 February 2013 Downloaded from https://academic.oup.com/cardiovascres/article/98/2/169/278625 by guest on 23 September 2021 Abstract This review highlights recent and ongoing discoveries that are transforming the previously held view of dyad structure and function. New data show that dyads vary greatly in both structure and in their associated molecules. Dyads can contain varying numbers of type 2 ryanodine receptor (RYR2) clusters that range in size from one to hundreds of tetramers and they can adopt numerous orientations other than the expected checkerboard. The association of Cav1.2 with RYR2, which defines the couplon, is not absolute, leading to a number of scenarios such as dyads without couplons and those in which only a fraction of the clusters are in couplons. Different dyads also vary in the transporters and exchangers with which they are associated producing functional differences that amplify their structural diversity. The essential role of proteins, such as junctophilin-2, calsequestrin, triadin, and junctin that main- tain both the functional and structural integrity of the dyad have recently been elucidated giving a new mechanistic understanding of heart diseases, such as arrhythmias, hypertension, failure, and sudden cardiac death. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Difference in Allelic Expression of the CLCN1 Gene and the Possible Influence on the Myotonia Congenita Phenotype
European Journal of Human Genetics (2004) 12, 738–743 & 2004 Nature Publishing Group All rights reserved 1018-4813/04 $30.00 www.nature.com/ejhg ARTICLE Difference in allelic expression of the CLCN1 gene and the possible influence on the myotonia congenita phenotype Morten Dun1*, Eskild Colding-Jrgensen2, Morten Grunnet3,5, Thomas Jespersen3, John Vissing4 and Marianne Schwartz1 1Department of Clinical Genetics, 4062, University Hospital, Rigshospitalet, Blegdamsvej 9, DK-2100 Copenhagen, Denmark; 2Department of Clinical Neurophysiology 3063,University Hospital, Rigshospitalet, Blegdamsvej 9, DK- 2100 Copenhagen, Denmark; 3Department of Medical Physiology, The Panum Institute, University of Copenhagen, Blegdamsvej 3, DK-2200 Copenhagen N, Denmark; 4Department of Neurology and The Copenhagen Muscle Research Center, University Hospital, Rigshospitalet, Blegdamsvej 9, DK-2100 Copenhagen, Denmark Mutations in the CLCN1 gene, encoding a muscle-specific chloride channel, can cause either recessive or dominant myotonia congenita (MC). The recessive form, Becker’s myotonia, is believed to be caused by two loss-of-function mutations, whereas the dominant form, Thomsen’s myotonia, is assumed to be a consequence of a dominant-negative effect. However, a subset of CLCN1 mutations can cause both recessive and dominant MC. We have identified two recessive and two dominant MC families segregating the common R894X mutation. Real-time quantitative RT-PCR did not reveal any obvious association between the total CLCN1 mRNA level in muscle and the mode of inheritance, but the dominant family with the most severe phenotype expressed twice the expected amount of the R894X mRNA allele. Variation in allelic expression has not previously been described for CLCN1, and our finding suggests that allelic variation may be an important modifier of disease progression in myotonia congenita. -

Back-To-Basics: the Intricacies of Muscle Contraction
Back-to- MIOTA Basics: The CONFERENCE OCTOBER 11, Intricacies 2019 CHERI RAMIREZ, MS, of Muscle OTRL Contraction OBJECTIVES: 1.Review the anatomical structure of a skeletal muscle. 2.Review and understand the process and relationship between skeletal muscle contraction with the vital components of the nervous system, endocrine system, and skeletal system. 3.Review the basic similarities and differences between skeletal muscle tissue, smooth muscle tissue, and cardiac muscle tissue. 4.Review the names, locations, origins, and insertions of the skeletal muscles found in the human body. 5.Apply the information learned to enhance clinical practice and understanding of the intricacies and complexity of the skeletal muscle system. 6.Apply the information learned to further educate clients on the importance of skeletal muscle movement, posture, and coordination in the process of rehabilitation, healing, and functional return. 1. Epithelial Four Basic Tissue Categories 2. Muscle 3. Nervous 4. Connective A. Loose Connective B. Bone C. Cartilage D. Blood Introduction There are 3 types of muscle tissue in the muscular system: . Skeletal muscle: Attached to bones of skeleton. Voluntary. Striated. Tubular shape. Cardiac muscle: Makes up most of the wall of the heart. Involuntary. Striated with intercalated discs. Branched shape. Smooth muscle: Found in walls of internal organs and walls of vascular system. Involuntary. Non-striated. Spindle shape. 4 Structure of a Skeletal Muscle Skeletal Muscles: Skeletal muscles are composed of: • Skeletal muscle tissue • Nervous tissue • Blood • Connective tissues 5 Connective Tissue Coverings Connective tissue coverings over skeletal muscles: .Fascia .Tendons .Aponeuroses 6 Fascia: Definition: Layers of dense connective tissue that separates muscle from adjacent muscles, by surrounding each muscle belly. -

Muscle Physiology Dr
Muscle Physiology Dr. Ebneshahidi Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Skeletal Muscle Figure 9.2 (a) Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Functions of the muscular system . 1. Locomotion . 2. Vasoconstriction and vasodilatation- constriction and dilation of blood vessel Walls are the results of smooth muscle contraction. 3. Peristalsis – wavelike motion along the digestive tract is produced by the Smooth muscle. 4. Cardiac motion . 5. Posture maintenance- contraction of skeletal muscles maintains body posture and muscle tone. 6. Heat generation – about 75% of ATP energy used in muscle contraction is released as heat. Copyright. © 2004 Pearson Education, Inc., publishing as Benjamin Cummings . Striation: only present in skeletal and cardiac muscles. Absent in smooth muscle. Nucleus: smooth and cardiac muscles are uninculcated (one nucleus per cell), skeletal muscle is multinucleated (several nuclei per cell ). Transverse tubule ( T tubule ): well developed in skeletal and cardiac muscles to transport calcium. Absent in smooth muscle. Intercalated disk: specialized intercellular junction that only occurs in cardiac muscle. Control: skeletal muscle is always under voluntary control‚ with some exceptions ( the tongue and pili arrector muscles in the dermis). smooth and cardiac muscles are under involuntary control. Copyright © 2004 Pearson Education, Inc., publishing as Benjamin Cummings Innervation: motor unit . a) a motor nerve and a myofibril from a neuromuscular junction where gap (called synapse) occurs between the two structures. at the end of motor nerve‚ neurotransmitter (i.e. acetylcholine) is stored in synaptic vesicles which will release the neurotransmitter using exocytosis upon the stimulation of a nerve impulse. Across the synapse the surface the of myofibril contains receptors that can bind with the neurotransmitter. -

Calcium Signaling and Cardiac Arrhythmias
Review Calcium Signaling Series Donald M. Bers, Guest Editor Calcium Signaling and Cardiac Arrhythmias Andrew P. Landstrom, Dobromir Dobrev, Xander H.T. Wehrens Abstract: There has been a significant progress in our understanding of the molecular mechanisms by which calcium (Ca2+) ions mediate various types of cardiac arrhythmias. A growing list of inherited gene defects can cause potentially lethal cardiac arrhythmia syndromes, including catecholaminergic polymorphic ventricular Downloaded from tachycardia, congenital long QT syndrome, and hypertrophic cardiomyopathy. In addition, acquired deficits of multiple Ca2+-handling proteins can contribute to the pathogenesis of arrhythmias in patients with various types of heart disease. In this review article, we will first review the key role of Ca2+ in normal cardiac function—in particular, excitation–contraction coupling and normal electric rhythms. The functional involvement of Ca2+ in distinct arrhythmia mechanisms will be discussed, followed by various inherited arrhythmia syndromes caused 2+ http://circres.ahajournals.org/ by mutations in Ca -handling proteins. Finally, we will discuss how changes in the expression of regulation of Ca2+ channels and transporters can cause acquired arrhythmias, and how these mechanisms might be targeted for therapeutic purposes. (Circ Res. 2017;120:1969-1993. DOI: 10.1161/CIRCRESAHA.117.310083.) Key Words: arrhythmias, cardiac ■ atrial fibrillation ■ calcium channels ■ cardiomyopathy ■ ryanodine receptor calcium release channel 2+ by guest on June 11, 2017 he bivalent cation calcium (Ca ) represents one of the Overview of Excitation–Contraction Tmost ubiquitous signal transduction molecules known.1 Coupling in the Heart It mediates a diverse array of biological functions including Regular contraction of the heart requires the conversion of muscle contraction, cellular exocytosis, neuronal activity, and electric activation (excitation) into mechanical force (con- triggering of programmed cell death. -

Comprehensive Exonic Sequencing of Known Ataxia Genes in Episodic Ataxia
biomedicines Article Comprehensive Exonic Sequencing of Known Ataxia Genes in Episodic Ataxia Neven Maksemous, Heidi G. Sutherland, Robert A. Smith, Larisa M. Haupt and Lyn R. Griffiths * Genomics Research Centre, Institute of Health and Biomedical Innovation (IHBI), School of Biomedical Sciences, Queensland University of Technology (QUT), Q Block, 60 Musk Ave, Kelvin Grove Campus, Brisbane, Queensland 4059, Australia; [email protected] (N.M.); [email protected] (H.G.S.); [email protected] (R.A.S.); [email protected] (L.M.H.) * Correspondence: lyn.griffi[email protected]; Tel.: +61-7-3138-6100 Received: 4 May 2020; Accepted: 21 May 2020; Published: 25 May 2020 Abstract: Episodic Ataxias (EAs) are a small group (EA1–EA8) of complex neurological conditions that manifest as incidents of poor balance and coordination. Diagnostic testing cannot always find causative variants for the phenotype, however, and this along with the recently proposed EA type 9 (EA9), suggest that more EA genes are yet to be discovered. We previously identified disease-causing mutations in the CACNA1A gene in 48% (n = 15) of 31 patients with a suspected clinical diagnosis of EA2, and referred to our laboratory for CACNA1A gene testing, leaving 52% of these cases (n = 16) with no molecular diagnosis. In this study, whole exome sequencing (WES) was performed on 16 patients who tested negative for CACNA1A mutations. Tiered analysis of WES data was performed to first explore (Tier-1) the ataxia and ataxia-associated genes (n = 170) available in the literature and databases for comprehensive EA molecular genetic testing; we then investigated 353 ion channel genes (Tier-2). -
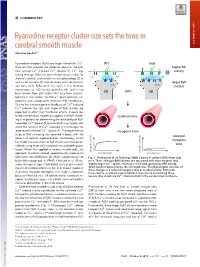
Ryanodine Receptor Cluster Size Sets the Tone in Cerebral Smooth Muscle
COMMENTARY Ryanodine receptor cluster size sets the tone in cerebral smooth muscle COMMENTARY Christian Soellera,1 + Ryanodine receptors (RyRs) are large intracellular Ca2 WT mdx channels that provide the molecular basis of the pro- K+ K+ higher BK + + + cess termed Ca2 -induced Ca2 release (1). Ca2 sig- activity naling through RyRs has been shown to be critical for CaC BK BK skeletal, cardiac, and smooth muscle physiology (2) as well as for neurons (3) and secretory cells like pancre- larger RyR atic beta cells. RyRs were first seen in the electron RyRs RyRs clusters microscope as ∼30-nm-size particles (4), and it had SMCs been known from EM studies that they form clusters, SR SR typically in the narrow “junctional” space between sar- colemma and sarcoplasmic reticulum (SR) membranes. + Due to the inherent positive feedback of Ca2 -induced + Ca2 release, the size and shape of RyR clusters are expected to affect their functional activity. Indeed, de- SMCs SMCs tailed mathematical modeling suggests that RyR cluster- cerebral artery ing is important for determining the excitability of RyR- + mediated Ca2 release (5, 6) and could, in principle, also + affect the amount of Ca2 released in microscopic re- 2+ lease events termed Ca sparks (7). The experimental myogenic tone study of RyR clustering has received a boost with the reduced advent of optical superresolution microscopy, which myogenic has made the assessment of RyR cluster size more acces- sible by using essentially standard immunolabeling pro- tone tocols. When first applied in cardiac muscle cells, this myogenic tone (%) tone myogenic approach revealed a broad, approximately exponential vessel pressure (%) tone myogenic vessel pressure RyR cluster size distribution (8), which had previously not Fig.