Kava As a Clinical Nutrient: Promises and Challenges
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
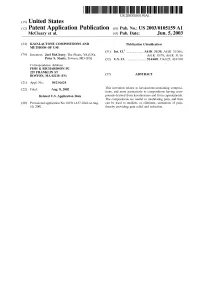
(12) Patent Application Publication (10) Pub. No.: US 2003/0105159 A1 Mccleary Et Al
US 200301 05159A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2003/0105159 A1 McCleary et al. (43) Pub. Date: Jun. 5, 2003 (54) KAVALACTONE COMPOSITIONS AND Publication Classification METHODS OF USE (51) Int. Cl." ....................... A61K 31/35; A61K 31/366; (76) Inventors: Joel McCleary, The Plains, VA (US); A61K 35/78; A61K 31/16 Peter S. Staats, Towson, MD (US) (52) U.S. Cl. ........................... 514/460; 514/625; 424/760 Correspondence Address: FISH & RICHARDSON PC 225 FRANKLIN ST BOSTON, MA 02110 (US) (57) ABSTRACT (21) Appl. No.: 10/214,624 (22) Filed: Aug. 8, 2002 This invention relates tO kavalactone-containing composi tions, and more particularly to compositions having com Related U.S. Application Data pounds derived from kavalactones and from capsaicinoids. The compositions are useful in modulating pain, and thus (60) Provisional application No. 60/311,437, filed on Aug. can be used to mediate, or eliminate, Sensations of pain, 10, 2001. thereby providing pain relief and reduction. US 2003/0105159 A1 Jun. 5, 2003 KAVALACTONE COMPOSITIONS AND METHODS 0006. In one embodiment, the invention relates to an OF USE analgesic topical composition having: (a) a kavalactone; (b) capsaicinoid or Synthetic derivatives thereof; and (c) a CROSS-REFERENCE TO RELATED pharmaceutically acceptable carrier; wherein the weight APPLICATIONS ratio of(a):(b) is from 5000:1 to 1:2 (e.g., 800:1 to 1:1; 500:1 to 5:1). In other aspects, the composition includes an effec 0001. This application claims benefit of U.S. application tive amount of kavalactones, active kavalactones, or capsai Ser. -

Kava (Piper Methysticum) and Its Methysticin Constituents Protect Brain Tissue Against Ischemic Damage in Rodents
5 Refs: Arletti R et al, Stimulating property of Turnera diffusa and Pfaffia paniculata extracts on the sexual-behavior of male rats. Psychopharmacology 143(1), 15-19, 1999. Berger F, Handbuch der drogenkunde . Vol 2, Maudrich, Wien, 1950. Martinez M, Les plantas medicinales de Mexico . Cuarta Edicion Botas Mexico , p119, 1959. Tyler VE et al, Pharmacognosy , 9 th edition, Lea & Febiger, Philadelphia, 1988. KAVA ( Piper methysticum ) - A REVIEW The Kava plant (Piper methysticum) is a robust, well-branching and erect perennial shrub belonging to the pepper family (Piperaceae). The botanical origin remains unknown, although it is likely that early Polynesian explorers brought the plant with them from island to island. Numerous varieties of Kava exist, and today it is widely cultivated in several Pacific Island countries both for local use as well as the rapidly growing demand for pharmaceutical preparations. The dried rhizomes (roots) are normally used. The first description to the western world of the ceremonial use of an intoxicating beverage prepared from Kava was made by Captain James Cook following his Pacific voyage in 1768. The drink, prepared as an infusion in an elaborate manner after first chewing the root, is consumed on formal occasions or meetings of village elders and chiefs, as well as in reconciling with enemies and on a more social basis. It remains an important social custom in many Pacific Island countries today. Most of the islands of the Pacific possessed Kava prior to European contact, particularly those encompassed by Polynesia, Melanesia and Micronesia. After drinking the Kava beverage a pleasantly relaxed and sociable state develops, after which a deep and restful sleep occurs. -

Alternative Treatments for Depression and Anxiety
2019 PCB Conference: Strickland Benzodiazepines (BZDs), Herbal and Alternative Treatments for Anxiety & Depression BZD Learning Objectives • List at least three uses for benzodiazepines • Discuss at least two risk factors associated with benzodiazepine prescriptions Craig Strickland, PhD, Owner Biobehavioral Education and Consultation https://sites.google.com/site/bioedcon 1 2 BZD Pharmacokinetics Clinical Uses of BZDs Generic Name Trade Name Rapidity ½ Life Dose (mg) • Treat a variety of anxiety disorders alprazolam Xanax Intermediate Short 0.75-4 • Hypnotics • Muscle relaxants chlordiaze- Librium Intermediate Long 15-100 poxide • To produce anterograde amnesia clonazepam Klonopin Intermediate Long 0.5-4 • Alcohol & other CNS depressant withdrawal • Anti-convulsant therapy diazepam Valium Rapid Long 4-40 triazolam Halcion Intermediate Very short 0.125-0.5 temazepam Restoril Short Short 7.5-30 3 4 1 2019 PCB Conference: Strickland Issues with BZDs Herbal Medication and Alternative Therapies Used in the Treatment of Depression and Anxiety • Addictive potential • Confusion between “anti-anxiety” effects and the “warm-fuzzy) • Large dose ranges • Comparison of BZDs with medications like Buspar, etc. • They work, they work well and they work quickly 5 6 Alternative Tx. Learning Objectives Background Information on herbals: Natural does not necessarily mean “safe” • List several amino acid treatments for depression • Side-effects and adverse reactions • List at least three of the most common herbal – Herbal medications are “drugs” although -

(12) Patent Application Publication (10) Pub. No.: US 2016/017.4603 A1 Abayarathna Et Al
US 2016O174603A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2016/017.4603 A1 Abayarathna et al. (43) Pub. Date: Jun. 23, 2016 (54) ELECTRONIC VAPORLIQUID (52) U.S. Cl. COMPOSITION AND METHOD OF USE CPC ................. A24B 15/16 (2013.01); A24B 15/18 (2013.01); A24F 47/002 (2013.01) (71) Applicants: Sahan Abayarathna, Missouri City, TX 57 ABSTRACT (US); Michael Jaehne, Missouri CIty, An(57) e-liquid for use in electronic cigarettes which utilizes- a TX (US) vaporizing base (either propylene glycol, vegetable glycerin, (72) Inventors: Sahan Abayarathna, MissOU1 City,- 0 TX generallyor mixture at of a 0.001 the two) g-2.0 mixed g per with 1 mL an ratio. herbal The powder herbal extract TX(US); (US) Michael Jaehne, Missouri CIty, can be any of the following:- - - Kanna (Sceletium tortuosum), Blue lotus (Nymphaea caerulea), Salvia (Salvia divinorum), Salvia eivinorm, Kratom (Mitragyna speciosa), Celandine (21) Appl. No.: 14/581,179 poppy (Stylophorum diphyllum), Mugwort (Artemisia), Coltsfoot leaf (Tussilago farfara), California poppy (Eschscholzia Californica), Sinicuichi (Heimia Salicifolia), (22) Filed: Dec. 23, 2014 St. John's Wort (Hypericum perforatum), Yerba lenna yesca A rtemisia scoparia), CaleaCal Zacatechichihichi (Calea(Cal termifolia), Leonurus Sibericus (Leonurus Sibiricus), Wild dagga (Leono Publication Classification tis leonurus), Klip dagga (Leonotis nepetifolia), Damiana (Turnera diffiisa), Kava (Piper methysticum), Scotch broom (51) Int. Cl. tops (Cytisus scoparius), Valarien (Valeriana officinalis), A24B 15/16 (2006.01) Indian warrior (Pedicularis densiflora), Wild lettuce (Lactuca A24F 47/00 (2006.01) virosa), Skullcap (Scutellaria lateriflora), Red Clover (Trifo A24B I5/8 (2006.01) lium pretense), and/or combinations therein. -

Could Mycolactone Inspire New Potent Analgesics? Perspectives and Pitfalls
toxins Review Could Mycolactone Inspire New Potent Analgesics? Perspectives and Pitfalls 1 2 3, 4, , Marie-Line Reynaert , Denis Dupoiron , Edouard Yeramian y, Laurent Marsollier * y and 1, , Priscille Brodin * y 1 France Univ. Lille, CNRS, Inserm, CHU Lille, Institut Pasteur de Lille, U1019-UMR8204-CIIL-Center for Infection and Immunity of Lille, F-59000 Lille, France 2 Institut de Cancérologie de l’Ouest Paul Papin, 15 rue André Boquel-49055 Angers, France 3 Unité de Microbiologie Structurale, Institut Pasteur, CNRS, Univ. Paris, F-75015 Paris, France 4 Equipe ATIP AVENIR, CRCINA, INSERM, Univ. Nantes, Univ. Angers, 4 rue Larrey, F-49933 Angers, France * Correspondence: [email protected] (L.M.); [email protected] (P.B.) These three authors contribute equally to this work. y Received: 29 June 2019; Accepted: 3 September 2019; Published: 4 September 2019 Abstract: Pain currently represents the most common symptom for which medical attention is sought by patients. The available treatments have limited effectiveness and significant side-effects. In addition, most often, the duration of analgesia is short. Today, the handling of pain remains a major challenge. One promising alternative for the discovery of novel potent analgesics is to take inspiration from Mother Nature; in this context, the detailed investigation of the intriguing analgesia implemented in Buruli ulcer, an infectious disease caused by the bacterium Mycobacterium ulcerans and characterized by painless ulcerative lesions, seems particularly promising. More precisely, in this disease, the painless skin ulcers are caused by mycolactone, a polyketide lactone exotoxin. In fact, mycolactone exerts a wide range of effects on the host, besides being responsible for analgesia, as it has been shown notably to modulate the immune response or to provoke apoptosis. -

(12) United States Patent (10) Patent No.: US 9.421,180 B2 Zielinski Et Al
USOO9421 180B2 (12) United States Patent (10) Patent No.: US 9.421,180 B2 Zielinski et al. (45) Date of Patent: Aug. 23, 2016 (54) ANTIOXIDANT COMPOSITIONS FOR 6,203,817 B1 3/2001 Cormier et al. .............. 424/464 TREATMENT OF INFLAMMATION OR 6,323,232 B1 1 1/2001 Keet al. ............ ... 514,408 6,521,668 B2 2/2003 Anderson et al. ..... 514f679 OXIDATIVE DAMAGE 6,572,882 B1 6/2003 Vercauteren et al. ........ 424/451 6,805,873 B2 10/2004 Gaudout et al. ....... ... 424/401 (71) Applicant: Perio Sciences, LLC, Dallas, TX (US) 7,041,322 B2 5/2006 Gaudout et al. .............. 424/765 7,179,841 B2 2/2007 Zielinski et al. .. ... 514,474 (72) Inventors: Jan Zielinski, Vista, CA (US); Thomas 2003/0069302 A1 4/2003 Zielinski ........ ... 514,452 Russell Moon, Dallas, TX (US); 2004/0037860 A1 2/2004 Maillon ...... ... 424/401 Edward P. Allen, Dallas, TX (US) 2004/0091589 A1 5, 2004 Roy et al. ... 426,265 s s 2004/0224004 A1 1 1/2004 Zielinski ..... ... 424/442 2005/0032882 A1 2/2005 Chen ............................. 514,456 (73) Assignee: Perio Sciences, LLC, Dallas, TX (US) 2005, 0137205 A1 6, 2005 Van Breen ..... 514,252.12 2005. O154054 A1 7/2005 Zielinski et al. ............. 514,474 (*) Notice: Subject to any disclaimer, the term of this 2005/0271692 Al 12/2005 Gervasio-Nugent patent is extended or adjusted under 35 et al. ............................. 424/401 2006/0173065 A1 8/2006 BeZwada ...................... 514,419 U.S.C. 154(b) by 19 days. 2006/O193790 A1 8/2006 Doyle et al. -

Anti-Inflammatory Activity of Compounds from Kaempferia Marginata Rhizomes
Songklanakarin J. Sci. Technol. 39 (1), 91-99, Jan. - Feb. 2017 http://www.sjst.psu.ac.th Original Article Anti-inflammatory activity of compounds from Kaempferia marginata rhizomes Kanidta Kaewkroek1, Chatchai Wattanapiromsakul1, 2, Hisashi Matsuda3, Seikou Nakamura3, and Supinya Tewtrakul1, 2* 1 Department of Pharmacognosy and Pharmaceutical Botany, Faculty of Pharmaceutical Sciences, Prince of Songkla University, Hat Yai, Songkhla, 90112 Thailand 2 Excellent Research Laboratory, Phytomedicine and Phamaceutical Biotechnology Excellence Center, Faculty of Pharmaceutical Sciences, Prince of Songkla University, Hat Yai, Songkhla, 90112 Thailand 3 Kyoto Pharmaceutical University, Misasagi, Yamashina-ku, Kyoto, 607-8412 Japan Received: 12 January 2016; Revised: 12 April 2016; Accepted: 19 April 2016 Abstract Two new pimarane diterpenes were obtained from Kaempferia marginata rhizomes, which are 1-acetoxysandara- copimaradien-2-one (1) and 1-acetoxysandaracopimaradiene (4), along with seven known compounds from the hexane and chloroform fractions including two pimarane-type diterpenes [marginatol (5), sandaracopimaradiene (8)], one kavalactone [desmethoxyyangonin (3)], three steroids [sitosterol--D-glucoside (2), the mixture of stigmasterol and -sitosterol (6 + 7)] and one diarylheptanoid [bisdemethoxycurcumin (9)]. Compounds 3 and 9 exhibited potent effect against NO production with IC50 of 10.1 and 6.8 µM, respectively. Compound 3 inhibited iNOS mRNA expression in a dose-dependent manner, while 9 suppressed both of iNOS and COX-2 genes. Moreover, compounds 2, 3, 6 + 7 and 9 were isolated for the first time from K. marginata. These results revealed that diterpenes, diarylheptanoid and kavalactone are components of K. marginata that afford anti-inflammatory effect through a mechanism involving a decrease in inflammatory mediators. Keywords: Kaempferia marginata, diterpenes, diarylheptanoid, kavalactone, anti-inflammatory activity 1. -

Herbal Insomnia Medications That Target Gabaergic Systems: a Review of the Psychopharmacological Evidence
Send Orders for Reprints to [email protected] Current Neuropharmacology, 2014, 12, 000-000 1 Herbal Insomnia Medications that Target GABAergic Systems: A Review of the Psychopharmacological Evidence Yuan Shia, Jing-Wen Donga, Jiang-He Zhaob, Li-Na Tanga and Jian-Jun Zhanga,* aState Key Laboratory of Bioactive Substance and Function of Natural Medicines, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, P.R. China; bDepartment of Pharmacology, School of Marine, Shandong University, Weihai, P.R. China Abstract: Insomnia is a common sleep disorder which is prevalent in women and the elderly. Current insomnia drugs mainly target the -aminobutyric acid (GABA) receptor, melatonin receptor, histamine receptor, orexin, and serotonin receptor. GABAA receptor modulators are ordinarily used to manage insomnia, but they are known to affect sleep maintenance, including residual effects, tolerance, and dependence. In an effort to discover new drugs that relieve insomnia symptoms while avoiding side effects, numerous studies focusing on the neurotransmitter GABA and herbal medicines have been conducted. Traditional herbal medicines, such as Piper methysticum and the seed of Zizyphus jujuba Mill var. spinosa, have been widely reported to improve sleep and other mental disorders. These herbal medicines have been applied for many years in folk medicine, and extracts of these medicines have been used to study their pharmacological actions and mechanisms. Although effective and relatively safe, natural plant products have some side effects, such as hepatotoxicity and skin reactions effects of Piper methysticum. In addition, there are insufficient evidences to certify the safety of most traditional herbal medicine. In this review, we provide an overview of the current state of knowledge regarding a variety of natural plant products that are commonly used to treat insomnia to facilitate future studies. -

Central Valley Toxicology Drug List
Chloroform ~F~ Lithium ~A~ Chlorpheniramine Loratadine Famotidine Acebutolol Chlorpromazine Lorazepam Fenoprofen Acetaminophen Cimetidine Loxapine Fentanyl Acetone Citalopram LSD (Lysergide) Fexofenadine 6-mono- Clomipramine acetylmorphine Flecainide ~M~ Clonazepam a-Hydroxyalprazolam Fluconazole Maprotiline Clonidine a-Hydroxytriazolam Flunitrazepam MDA Clorazepate Albuterol Fluoxetine MDMA Clozapine Alprazolam Fluphenazine Medazepam Cocaethylene Amantadine Flurazepam Meperidine Cocaine 7-Aminoflunitrazepam Fluvoxamine Mephobarbital Codeine Amiodarone Fosinopril Meprobamate Conine Amitriptyline Furosemide Mesoridazine Cotinine Amlodipine Methadone Cyanide ~G~ Amobarbital Methanol Cyclobenzaprine Gabapentin Amoxapine d-Methamphetamine Cyclosporine GHB d-Amphetamine l-Methamphetamine Glutethamide l-Amphetamine ~D~ Methapyrilene Guaifenesin Aprobarbital Demoxepam Methaqualone Atenolol Desalkylfurazepam ~H~ Methocarbamol Atropine Desipramine Halazepam Methylphenidate ~B~ Desmethyldoxepin Haloperidol Methyprylon Dextromethoraphan Heroin Metoclopramide Baclofen Diazepam Hexobarbital Metoprolol Barbital Digoxin Hydrocodone Mexiletine Benzoylecgonine Dihydrocodein Hydromorphone Midazolam Benzphetamine Dihydrokevain Hydroxychloroquine Mirtazapine Benztropine Diltiazem Hydroxyzine Morphine (Total/Free) Brodificoum Dimenhydrinate Bromazepam ~N~ Diphenhydramine ~I~ Bupivacaine Nafcillin Disopyramide Ibuprofen Buprenorphine Naloxone Doxapram Imipramine Bupropion Naltrexone Doxazosin Indomethacin Buspirone NAPA Doxepin Isoniazid Butabarbital Naproxen -

Herbal Medicines in Pregnancy and Lactation : an Evidence-Based
00 Prelims 1410 10/25/05 2:13 PM Page i Herbal Medicines in Pregnancy and Lactation An Evidence-Based Approach Edward Mills DPh MSc (Oxon) Director, Division of Clinical Epidemiology Canadian College of Naturopathic Medicine North York, Ontario, Canada Jean-Jacques Duguoa MSc (cand.) ND Naturopathic Doctor Toronto Western Hospital Assistant Professor Division of Clinical Epidemiology Canadian College of Naturopathic Medicine North York, Ontario, Canada Dan Perri BScPharm MD MSc Clinical Pharmacology Fellow University of Toronto Toronto, Ontario, Canada Gideon Koren MD FACMT FRCP Director of Motherisk Professor of Medicine, Pediatrics and Pharmacology University of Toronto Toronto, Ontario, Canada With a contribution from Paul Richard Saunders PhD ND DHANP 00 Prelims 1410 10/25/05 2:13 PM Page ii © 2006 Taylor & Francis Medical, an imprint of the Taylor & Francis Group First published in the United Kingdom in 2006 by Taylor & Francis Medical, an imprint of the Taylor & Francis Group, 2 Park Square, Milton Park, Abingdon, Oxon OX14 4RN Tel.: ϩ44 (0)20 7017 6000 Fax.: ϩ44 (0)20 7017 6699 E-mail: [email protected] Website: www.tandf.co.uk/medicine All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or trans- mitted, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without the prior permission of the publisher or in accordance with the provisions of the Copyright, Designs and Patents Act 1988 or under the terms of any licence permitting limited copying issued by the Copyright Licensing Agency, 90 Tottenham Court Road, London W1P 0LP. -

NIH Public Access Author Manuscript Pharmacol Ther
NIH Public Access Author Manuscript Pharmacol Ther. Author manuscript; available in PMC 2010 August 1. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: Pharmacol Ther. 2009 August ; 123(2): 239±254. doi:10.1016/j.pharmthera.2009.04.002. Ethnobotany as a Pharmacological Research Tool and Recent Developments in CNS-active Natural Products from Ethnobotanical Sources Will C. McClatcheya,*, Gail B. Mahadyb, Bradley C. Bennettc, Laura Shielsa, and Valentina Savod a Department of Botany, University of Hawaìi at Manoa, Honolulu, HI 96822, U.S.A b Department of Pharmacy Practice, University of Illinois at Chicago, Chicago, IL 60612, U.S.A c Department of Biological Sciences, Florida International University, Miami, FL 33199, U.S.A d Dipartimento di Biologia dì Roma Trè, Viale Marconi, 446, 00146, Rome, Italy Abstract The science of ethnobotany is reviewed in light of its multidisciplinary contributions to natural product research for the development of pharmaceuticals and pharmacological tools. Some of the issues reviewed involve ethical and cultural perspectives of healthcare and medicinal plants. While these are not usually part of the discussion of pharmacology, cultural concerns potentially provide both challenges and insight for field and laboratory researchers. Plant evolutionary issues are also considered as they relate to development of plant chemistry and accessing this through ethnobotanical methods. The discussion includes presentation of a range of CNS-active medicinal plants that have been recently examined in the field, laboratory and/or clinic. Each of these plants is used to illustrate one or more aspects about the valuable roles of ethnobotany in pharmacological research. -

Plant-Based Medicines for Anxiety Disorders, Part 2: a Review of Clinical Studies with Supporting Preclinical Evidence
CNS Drugs 2013; 24 (5) Review Article Running Header: Plant-Based Anxiolytic Psychopharmacology Plant-Based Medicines for Anxiety Disorders, Part 2: A Review of Clinical Studies with Supporting Preclinical Evidence Jerome Sarris,1,2 Erica McIntyre3 and David A. Camfield2 1 Department of Psychiatry, Faculty of Medicine, University of Melbourne, Richmond, VIC, Australia 2 The Centre for Human Psychopharmacology, Swinburne University of Technology, Melbourne, VIC, Australia 3 School of Psychology, Charles Sturt University, Wagga Wagga, NSW, Australia Correspondence: Jerome Sarris, Department of Psychiatry and The Melbourne Clinic, University of Melbourne, 2 Salisbury Street, Richmond, VIC 3121, Australia. Email: [email protected], Acknowledgements Dr Jerome Sarris is funded by an Australian National Health & Medical Research Council fellowship (NHMRC funding ID 628875), in a strategic partnership with The University of Melbourne, The Centre for Human Psychopharmacology at the Swinburne University of Technology. Jerome Sarris, Erica McIntyre and David A. Camfield have no conflicts of interest that are directly relevant to the content of this article. 1 Abstract Research in the area of herbal psychopharmacology has revealed a variety of promising medicines that may provide benefit in the treatment of general anxiety and specific anxiety disorders. However, a comprehensive review of plant-based anxiolytics has been absent to date. Thus, our aim was to provide a comprehensive narrative review of plant-based medicines that have clinical and/or preclinical evidence of anxiolytic activity. We present the article in two parts. In part one, we reviewed herbal medicines for which only preclinical investigations for anxiolytic activity have been performed. In this current article (part two), we review herbal medicines for which there have been both preclinical and clinical investigations for anxiolytic activity.