Information to Users
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thesis Submitted By
University of Bath PHD A study of low voltage polyacrylamide gel electrophoresis as a means of providing controlled drug release Kumar, Ravinder Award date: 1986 Awarding institution: University of Bath Link to publication Alternative formats If you require this document in an alternative format, please contact: [email protected] General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. • Users may download and print one copy of any publication from the public portal for the purpose of private study or research. • You may not further distribute the material or use it for any profit-making activity or commercial gain • You may freely distribute the URL identifying the publication in the public portal ? Take down policy If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Download date: 11. Oct. 2021 A Study of Low Voltage Polyacrylamide Gel Electrophoresis as a means of providing Controlled Drug Release A thesis submitted by Ravlnder Kumar for the degree of Doctor of Philosophy of the University of Bath 19 86 Copyright Attention is drawn to the fact that copyright, of this thesis rests with its author. A copy of this thesis has been supplied on condition that anyone who consults it is understood to recognise that its copyright rests with its author and that no quotation from the thesis and no information derived from it may be published without the prior written consent of the author. -

Associated Problems of Protein Electrophoresis, Staining and Densitometry*
ANNALS OF CLINICAL AND LABORATORY SCIENCE, Vol. 8, No. 5 Copyright © 1978, Institute for Clinical Science Associated Problems of Protein Electrophoresis, Staining and Densitometry* BENNIE ZAK, Ph.D.,f EUGENE S. BAGINSKI, Ph.D.,* and EMANUEL EPSTEIN, Ph.D.§ Departments of Clinical Pathology: t Wayne State University School of Medicine and Detroit General Hospital, Detroit, MI 48201 f St. Joseph Mercy Hospital, Pontiac, Ml 48053 § William Beaumont Hospital, Royal Oak, MI 48072 ABSTRACT The process of electrophoresis, a separation phenomenon, is mistakenly understood to include the sequential processes ancillary to analyte resolu tion, that is, staining and quantification, where the latter could be elution followed by photometry or integrating-calculating-densitometry. The theories involved in electrophoresis itself are well worked OuFand equally well understood but the problems which are associated with separation, chemical reaction to generate a chromogen and quantification, perhaps partly forgotten and perhaps partly ignored, are taken up and described here. They include albumin trail, resolution, unequivalent staining, prestaining and the densitometry problems associated with band widths, opacity effects and polychromaticities. Introduction and perfected of the three phases in terms of accomplishing its purpose with the Any discussion of protein electro least problems, that is to separate proteins phoresis should include electrophoresis into several well resolved zones by the itself, and the sequential phases as simplest means possible. -

(CFTR) Allelic Variants Relate to Shifts in Faecal Microbiota of Cystic Fibrosis Patients
Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Allelic Variants Relate to Shifts in Faecal Microbiota of Cystic Fibrosis Patients Serena Schippa1., Valerio Iebba1*., Floriana Santangelo1, Antonella Gagliardi1, Riccardo Valerio De Biase2, Antonella Stamato2, Serenella Bertasi2, Marco Lucarelli3, Maria Pia Conte1", Serena Quattrucci2" 1 Public Health and Infectious Diseases Department, Microbiology Unit, ‘Sapienza’ University of Rome, Rome, Italy, 2 Regional Cystic Fibrosis Centre, Paediatrics and Infant Neuropsychiatry Department, ‘Sapienza’ University of Rome, Rome, Italy, 3 Department of Haematology and Cellular Biotechnologies, Sapienza University of Rome, Rome, Italy Abstract Introduction: In this study we investigated the effects of the Cystic Fibrosis Transmembrane conductance Regulator (CFTR) gene variants on the composition of faecal microbiota, in patients affected by Cystic Fibrosis (CF). CFTR mutations (F508del is the most common) lead to a decreased secretion of chloride/water, and to mucus sticky secretions, in pancreas, respiratory and gastrointestinal tracts. Intestinal manifestations are underestimated in CF, leading to ileum meconium at birth, or small bowel bacterial overgrowth in adult age. Methods: Thirty-six CF patients, fasting and under no-antibiotic treatment, were CFTR genotyped on both alleles. Faecal samples were subjected to molecular microbial profiling through Temporal Temperature Gradient Electrophoresis and species-specific PCR. Ecological parameters and multivariate algorithms were employed to find out if CFTR variants could be related to the microbiota structure. Results: Patients were classified by two different criteria: 1) presence/absence of F508del mutation; 2) disease severity in heterozygous and homozygous F508del patients. We found that homozygous-F508del and severe CF patients exhibited an enhanced dysbiotic faecal microbiota composition, even within the CF cohort itself, with higher biodiversity and evenness. -

Microalgae Amino Acid Extraction and Analysis At
Microalgae amino acid extraction and analysis at nanomolar level using electroporation and capillary electrophoresis with laser-induced fluorescence detection Reine Nehmé, Carla Atieh, Syntia Fayad, Bérengère Claude, Agnès Chartier, Mona Tannoury, Fatma Elleuch, Slim Abdelkafi, Chantal Pichon, Philippe Morin To cite this version: Reine Nehmé, Carla Atieh, Syntia Fayad, Bérengère Claude, Agnès Chartier, et al.. Microalgae amino acid extraction and analysis at nanomolar level using electroporation and capillary electrophoresis with laser-induced fluorescence detection. Journal of Separation Science, Wiley-VCH Verlag, 2017,40 (2), pp.558-566. 10.1002/jssc.201601005. hal-01618682 HAL Id: hal-01618682 https://hal.archives-ouvertes.fr/hal-01618682 Submitted on 25 Jun 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License Microalgae amino acid extraction and analysis at nanomolar level using electroporation and capillary electrophoresis with laser-induced fluorescence detection Reine -

Determination and Implications of Electroosmotic Transport In
DETERMINATION AND IMPLICATIONS OF ELECTROOSMOTIC TRANSPORT IN THE BRAIN by Yifat Guy BS, University of Pittsburgh, 2004 Submitted to the Graduate Faculty of Arts and Sciences in partial fulfillment of the requirements for the degree of Doctor of Philosophy University of Pittsburgh 2011 UNIVERSITY OF PITTSBURGH Graduate Arts and Sciences This dissertation was presented by Yifat Guy It was defended on December 15, 2010 and approved by Dr. Shigeru Amemiya, Professor, Department of Chemistry Dr. Adrian Michael, Professor, Department of Chemistry Dr. Mats Sandberg, Professor, Department of Anatomy and Cell Biology, Gothenberg University Dissertation Advisor: Dr. Stephen G. Weber, Professor, Department of Chemistry ii Copyright © by Yifat Guy 2011 Reproduced with permission from: Guy Y, Sandberg M, Weber SG. Biophysical Journal. 2008;94(11):4561-4569. Guy Y, Muha RJ, Sandberg M, Weber SG. Analytical Chemistry. 2009;81(8):3001-3007. iii DETERMINATION AND IMPLICATIONS OF ELECTROOSMOTIC TRANSPORT IN THE BRAIN Yifat Guy, PhD University of Pittsburgh, 2011 Electroosmotic flow is a bulk fluid flow that influences solute transport through capillary conduits and porous material under the influence of an electric field. The magnitude of electroosmotic flow is proportional to the ζ-potential of the capillary or porous material. A porous material such as living tissue would need to have an appreciable ζ-potential to create electroosmotic flow in the interstitial space. Transporting solutes in and out of tissue by virtue of electroosmotic flow in principle has advantages such as avoiding pressure effects and sample dilution that accompany micropush-pull and microdialysis approaches. In order to assess the viability of this approach, it is necessary to know the ζ-potential in the tissue of interest. -

WO 2018/035147 Al 22 February 2018 (22.02.2018) W !P O PCT
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2018/035147 Al 22 February 2018 (22.02.2018) W !P O PCT (51) International Patent Classification: (74) Agent: WORD, Michael, J.; Mayer Brown LLP, P.O. Box G06F 17/00 (2006.01) 2828, Chicago, IL 60690-2828 (US). (21) International Application Number: (81) Designated States (unless otherwise indicated, for every PCT/US20 17/046997 kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, BZ, (22) International Filing Date: CA, CH, CL, CN, CO, CR, CU, CZ, DE, DJ, DK, DM, DO, 15 August 2017 (15.08.2017) DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, (25) Filing Language: English HR, HU, ID, IL, IN, IR, IS, JO, JP, KE, KG, KH, KN, KP, KR, KW, KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, (26) Publication Langi English MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (30) Priority Data: OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, 62/375,256 15 August 2016 (15.08.2016) US SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, 62/375,192 15 August 2016 (15.08.2016) US TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. 62/416,972 03 November 2016 (03 .11.20 16) US (84) Designated States (unless otherwise indicated, for every 62/427,919 30 November 2016 (30. -

Pain Relief for the Peripheral Venous Cannulation Of
Bond et al. BMC Anesthesiology (2016) 16:81 DOI 10.1186/s12871-016-0252-8 RESEARCH ARTICLE Open Access First do no harm: pain relief for the peripheral venous cannulation of adults, a systematic review and network meta- analysis Mary Bond1, Louise Crathorne1* , Jaime Peters1, Helen Coelho1, Marcela Haasova1, Chris Cooper1, Quentin Milner2, Vicki Shawyer3, Christopher Hyde1 and Roy Powell4 Abstract Background: Peripheral venous cannulation is an everyday practice in hospitals, which many adults find painful. However, anaesthesia for cannulation is usually only offered to children. Inadequate pain relief is not only unpleasant for patients but may cause anxiety about further treatment and deter patients from seeking medical care in the future. The aim of this study is to discover the most effective local anaesthetic for adult peripheral venous cannulation and to find out how the pain of local anaesthetic application compares with that of unattenuated cannulation. Methods: These aims are addressed through a systematic review, network meta-analysis and random-effects meta-analysis. Searching covered 12 databases including MEDLINE and EMBASE from 1990 to August 2015. The main included study design was RCTs. The primary outcome measure is self-reported pain, measured on a 100 mm visual analogue scale. Results: The systematic review found 37 includable studies, 27 of which were suitable for network meta-analysis and two for random-effects meta-analysis. The results of the network meta-analysis indicate that none of the 17 anaesthetic considered had a very high probability of being the most effective when compared to each other; 2 % lidocaine had the highest probability (44 %). -

204200Orig1s000 204200Orig2s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 204200Orig1s000 204200Orig2s000 MEDICAL REVIEW(S) CLINICAL REVIEW Application Type NDA Application Number(s) 204-200 and 204-640 Priority or Standard Standard Submit Date(s) March 7, 2012 Received Date(s) March 7, 2012 PDUFA Goal Date January 7, 2013 Division / Office DPARP/OND Reviewer Name(s) Peter Starke, MD Review Completion Date October 29, 2012 Established Name Epinephrine injection, USP, 1mg/mL (Proposed) Trade Name Adrenalin® Therapeutic Class Catecholamine Applicant JHP Pharmaceuticals, LLC Formulation(s) Solution for injection Proposed Dosing Hypersensitivity reactions: IM or SC Regimen injection(b) (4) [Ophthalmic use: Topical irrigation or intraocular bolus injection] Proposed Indication(s) Hypersensitivity reactions: severe acute anaphylactic reactions, (b) (4) [Ophthalmic use: induction and maintenance of mydriasis during cataract surgery] Intended Population(s) Hypersensitivity reactions: No age restrictions [Ophthalmic use: No age restrictions] Reference ID: 3209828 Clinical Review ● Peter Starke, MD NDA 204-200 ● Adrenalin® (epinephrine) MEDICAL OFFICER REVIEW Division of Pulmonary, Allergy and Rheumatology Products (DPARP) SUBMISSIONS REVIEWED IN THIS DOCUMENT Document Date CDER Stamp Date Submission Comments March 7, 2012 March 7, 2012 SD-1, eCTD-0000 New NDA submission April 9, 2012 April 9, 2012 SD-2, eCTD-0001 PREA waiver request – Anaphylaxis April 9, 2012 April 9, 2012 SD-3, eCTD-0002 PREA waiver request – Mydriasis April 18, 2012 April 18, 2012 SD-4, eCTD-0003 Request for proprietary name review Sept 5, 2012 Sept 6, 2012 SD-22, eCTD-0021 Draft Ophthalmic Labeling Oct 22, 2012 Oct 22, 2012 SD-27, eCDT-0026 Certification that EpiPen is the reference for the 505(b)(2) application RECOMMENDED REGULATORY ACTION NDA/SUPPLEMENTS: X APPROVAL COMPLETE RESPONSE OTHER ACTION: 2 Reference ID: 3209828 Clinical Review ● Peter Starke, MD NDA 204-200 ● Adrenalin® (epinephrine) Table of Contents 1 RECOMMENDATIONS/RISK BENEFIT ASSESSMENT ........................................ -

Code of Federal Regulations GPO Access
4±28±97 Monday Vol. 62 No. 81 April 28, 1997 Pages 22873±23124 Briefings on how to use the Federal Register For information on briefings in Washington, DC, see announcement on the inside cover of this issue. For information on briefings in Kansas City and Independence, MO, Long Beach and San Francisco, CA, and Anchorage, AK, see the announcement in the Reader Aids. Now Available Online Code of Federal Regulations via GPO Access (Selected Volumes) Free, easy, online access to selected Code of Federal Regulations (CFR) volumes is now available via GPO Access, a service of the United States Government Printing Office (GPO). CFR titles will be added to GPO Access incrementally throughout calendar years 1996 and 1997 until a complete set is available. GPO is taking steps so that the online and printed versions of the CFR will be released concurrently. The CFR and Federal Register on GPO Access, are the official online editions authorized by the Administrative Committee of the Federal Register. New titles and/or volumes will be added to this online service as they become available. http://www.access.gpo.gov/nara/cfr For additional information on GPO Access products, services and access methods, see page II or contact the GPO Access User Support Team via: ★ Phone: toll-free: 1-888-293-6498 ★ Email: [email protected] federal register 1 II Federal Register / Vol. 62, No. 81 / Monday, April 28, 1997 SUBSCRIPTIONS AND COPIES PUBLIC Subscriptions: Paper or fiche 202±512±1800 Assistance with public subscriptions 512±1806 General online information 202±512±1530; 1±888±293±6498 FEDERAL REGISTER Published daily, Monday through Friday, Single copies/back copies: (not published on Saturdays, Sundays, or on official holidays), by the Office of the Federal Register, National Archives and Paper or fiche 512±1800 Records Administration, Washington, DC 20408, under the Federal Assistance with public single copies 512±1803 Register Act (49 Stat. -

United States Patent (10) Patent No.: US 9,463,201 B2 Alster Et Al
USOO94632O1B2 (12) United States Patent (10) Patent No.: US 9,463,201 B2 Alster et al. (45) Date of Patent: Oct. 11, 2016 (54) COMPOSITIONS AND METHODS FOR THE 2010/02851.55 A1 11, 2010 Gilbard et al. TREATMENT OF MEBOMAN GLAND 2011/0022O10 A1 1/2011 Grenon et al. 2011/0059925 A1 3/2011 Donnenfeld DYSFUNCTION 2011/O124725 A1* 5, 2011 Maskin ................ A61K 31, 167 514,529 (71) Applicant: M.G. Therapeutics, Ltd., Tel Aviv (IL) 2011 0130729 A1 6, 2011 Korb et al. 2011 0137214 A1 6, 2011 Korb et al. (72) Inventors: Yair Alster, Tel Aviv (IL); Omer 2011/0294.897 Al 12/2011 Aberg et al. Rafaeli. Udim (IL); K. Angela 2012fOO16275 A1 1/2012 Korb et al. Macf i M o Park, CA (US); 2012/0028929 A1 2/2012 Power et al. aciariane, Menlo Park, s 2012/0093876 Al 4/2012 Ousler, III et al. Cary Reich, Los Gatos, CA (US); 2012/O128763 A1 5, 2012 Maskin Shimon Amselem, Rehovot (IL); 2012/0190661 A1 7/2012 Trogden et al. Doron Friedman, Carme-Yosef (IL) 2012fO226156 A1 9/2012 Grenon et al. 2012fO264681 A1 10, 2012 Braiman-WikSman et al. 2012fO288575 A1 11, 2012 Gilbard et al. (73) Assignee: M.G. THERAPEUTICS LTD, Tel 2013,0053733 A1 2/2013 Korb et al. Aviv (IL) 2013,013 1171 A1 5.2013 Maskin 2013, O184242 A1 7/2013 Eini et al. (*) Notice: Subject to any disclaimer, the term of this 2013/0224272 A1 8/2013 Gao et al. patent is extended or adjusted under 35 3.855. A. -

Microelectrophoresis of Semiconductive Quantum Dots
Microelectrophoresis of Semiconductive Quantum Dots By Mengke Han A thesis submitted for the fulfilment of the degree of Master of Philosophy in the Faculty of Sciences School of Physical Sciences Aug 2017 Declaration of Authorship I certify that this work contains no material which has been accepted for the award of any other degree or diploma in my name in any university or other tertiary institution and, to the best of my knowledge and belief, contains no material previously published or written by another person, except where due reference has been made in the text. In addition, I certify that no part of this work will, in the future, be used in a submission in my name for any other degree or diploma in any university or other tertiary institution without the prior approval of the University of Adelaide and where applicable, any partner institution responsible for the joint award of this degree. I give consent to this copy of my thesis when deposited in the University Library, being made available for loan and photocopying, subject to the provisions of the Copyright Act 1968. I also give permission for the digital version of my thesis to be made available on the web, via the University's digital research repository, the Library Search and also through web search engines, unless permission has been granted by the University to restrict access for a period of time. Signed: Date: II Table of contents Declaration of Authorship .................................................................................. II Abstract .............................................................................................................. -
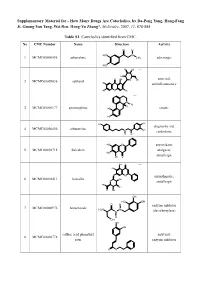
How Many Drugs Are Catecholics, by Da-Peng Yang, Hong-Fang Ji, Guang-Yan Tang, Wei Ren, Hong-Yu Zhang*, Molecules, 2007, 12, 878-884
Supplementary Material for - How Many Drugs Are Catecholics, by Da-Peng Yang, Hong-Fang Ji, Guang-Yan Tang, Wei Ren, Hong-Yu Zhang*, Molecules, 2007, 12, 878-884 Table S1. Catecholics identified from CMC. No CMC Number Name Structure Activity O H HO N 1 MCMC00000595 adrenalone CH3 adrenergic HO HO OH Chiral HO O OH antiviral; 2 MCMC00009836 aphloiol O OH HO antiinflammatory HO O OH Chiral N CH H 3 3 MCMC00000177 apomorphine HO emetic HO HO OH diagnostic aid; 4 MCMC00006456 arbutamine N OH cardiotonic H OH HO O antioxidant; 5 MCMC00010715 baicalein analgesic; HO OH O antiallergic OH O Chiral HO O O antiasthmatic; 6 MCMC00010411 baicalin OH O antiallergic HO OH O OH OH HO OH O H enzyme inhibitor 7 MCMC00000976 benserazide H2N N N (decarboxylase) H OH OH OH caffeic acid phenethyl antiviral; 8 MCMC00006778 ester enzyme inhibitor O O Molecules, 2007, 12, Molecules, 2007, 12, 878-884 2 Table S1. Cont. O OH enzyme inhibitor 9 MCMC00010571 carbidopa CH H N 3 (decarboxylase) HO NH2 OH HO OChiral O O N S N S antibiotic; 10 MCMC00006131 cefetecol H H H2N N N H antibacterial O O HO OH H HO O Chiral HO O 11 MCMC00010530 chf-1301 antiparkinsonian NH CH HO 2 3 OH Chiral HO antiviral; 12 MCMC00000898 cianidol HO O OH antineoplastic HO OH O O OH O O HO phagocyte stimulant; 13 MCMC00007713 cichoric acid O O OH HO antineoplastic HO HO O O HO 14 MCMC00009461 citromycetin O antibiotic HOO CH3 HO OH antispasmodic; 15 MCMC00004237 clofeverine O Cl anticholinergic N H H3C CH3 CH3 16 MCMC00003077 colterol N bronchodilator HO H OH HO HOHO O O 17 MCMC00001729 cynarine O choleretic HO O OH HO OH O OH Molecules, 2007, 12, Molecules, 2007, 12, 878-884 3 Table S1.