Exotic Medicinal Plants-Current Status and Future Priorities
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

State of Colorado 2016 Wetland Plant List
5/12/16 State of Colorado 2016 Wetland Plant List Lichvar, R.W., D.L. Banks, W.N. Kirchner, and N.C. Melvin. 2016. The National Wetland Plant List: 2016 wetland ratings. Phytoneuron 2016-30: 1-17. Published 28 April 2016. ISSN 2153 733X http://wetland-plants.usace.army.mil/ Aquilegia caerulea James (Colorado Blue Columbine) Photo: William Gray List Counts: Wetland AW GP WMVC Total UPL 83 120 101 304 FACU 440 393 430 1263 FAC 333 292 355 980 FACW 342 329 333 1004 OBL 279 285 285 849 Rating 1477 1419 1504 1511 User Notes: 1) Plant species not listed are considered UPL for wetland delineation purposes. 2) A few UPL species are listed because they are rated FACU or wetter in at least one Corps Region. 3) Some state boundaries lie within two or more Corps Regions. If a species occurs in one region but not the other, its rating will be shown in one column and the other column will be BLANK. Approved for public release; distribution is unlimited. 1/22 5/12/16 Scientific Name Authorship AW GP WMVC Common Name Abies bifolia A. Murr. FACU FACU Rocky Mountain Alpine Fir Abutilon theophrasti Medik. UPL UPL FACU Velvetleaf Acalypha rhomboidea Raf. FACU FACU Common Three-Seed-Mercury Acer glabrum Torr. FAC FAC FACU Rocky Mountain Maple Acer grandidentatum Nutt. FACU FAC FACU Canyon Maple Acer negundo L. FACW FAC FAC Ash-Leaf Maple Acer platanoides L. UPL UPL FACU Norw ay Maple Acer saccharinum L. FAC FAC FAC Silver Maple Achillea millefolium L. FACU FACU FACU Common Yarrow Achillea ptarmica L. -

List of Rare, Threatened, and Endangered Species of Dorchester County
List of Rare, Threatened, and Endangered Species of Dorchester County July 2019 Maryland Wildlife and Heritage Service Natural Heritage Program Larry Hogan, Governor Jeannie Haddaway-Riccio, Secretary Wildlife & Heritage Service Natural Heritage Program Tawes State Office Building, E-1 580 Taylor Avenue Annapolis, MD 21401 410-260-8540 Fax 410-260-8596 dnr.maryland.gov/wildlife Additional Telephone Contact Information: Toll free in Maryland: 877-620-8DNR ext. 8540 OR Individual unit/program toll-free number Out of state call: 410-260-8540 Text Telephone (TTY) users call via the Maryland Relay The facilities and services of the Maryland Department of Natural Resources are available to all without regard to race, color, religion, sex, sexual orientation, age, national origin or physical or mental disability. This document is available in alternative format upon request from a qualified individual with disability. ACKNOWLEDGMENTS The Maryland Department of Natural Resources would like to express sincere appreciation to the many scientists and naturalists who willingly share information and provide their expertise to further our mission of conserving Maryland’s natural heritage. Publication of this list is made possible by taxpayer donations to Maryland’s Chesapeake Bay and Endangered Species Fund. IMPORTANT NOTES This list is a subset of the main reports: Maryland Natural Heritage Program. 2019. List of Rare, Threatened, and Endangered Plants of Maryland DNR 03-031319-135 and Maryland Natural Heritage Program. 2019. Rare, Threatened, and Endangered Plants of Maryland DNR 03-031319-136 and Maryland Natural Heritage Program. 2016. List of Rare, Threatened, and Endangered Animals of Maryland DNR 03-1272016-633 Please refer to these for important information including grank, history, purpose, governing laws and regulations, understanding state and federal conservation status ranks and legal statuses, and for additional resources. -

1501:18-1-03 Endangered and Threatened Species
ACTION: Revised DATE: 10/22/2014 11:54 AM 1501:18-1-03 Endangered and threatened species. (A) The following species of plants are designated as endangered in Ohio. (1) Acer pensylvanicum L., Striped maple. (2) Aconitum noveboracense A. Gray, Northern monkshood. (3) Aconitum uncinatum L., Southern monkshood. (4) Agalinis auriculata (Michx.) Blake, Ear-leaved-foxglove. (5) Agalinis purpurea (L.) Pennell var. parviflora (Benth.) Boivin, Small purple-foxglove. (6) Agalinis skinneriana (Wood) Britt., Skinner's-foxglove. (7) Ageratina aromatica (L.) Spach, Small white snakeroot. (8) Agrostis elliottiana Schultes, Elliott's bent grass. (9) Amelanchier humilis Wiegand, Low serviceberry. (10) Amelanchier interior E.L. Nielsen, Inland serviceberry. (9)(11) Andropogon glomeratus (Walter) Britton, Bushy broom-sedge. (10)(12) Androsace occidentalis Pursh, Western rock-jasmine. (11)(13) Anomobryum filiforme (Dicks.) Solms, Common silver moss. (12)(14) Anomodon viticulosus (Hedw.) Hook. & Taylor, Long tail moss. (13)(15) Arabidopsis lyrata (Linnaeus) O’Kane & Al-Shehbaz, Lyre-leaved rock cress. (14)(16) Arabis patens Sullivant, Spreading rock cress. (15)(17) Arctostaphylos uva-ursi (L.) Spreng., Bearberry. (16)(18) Aralia hispida Vent., Bristly sarsaparilla. [ stylesheet: rule.xsl 2.14, authoring tool: i4i 2.0 ras3 May 23, 2014 10:53, (dv: 0, p: 120697, pa: 243620, ra: 421552, d: print date: 10/22/2014 08:00 PM 1501:18-1-03 2 (17)(19) Arethusa bulbosa L., Dragon's-mouth. (20) Aristida basiramea Engelm. ex Vasey, Forked Three-awn grass. (18)(21) Aristida necopina Shinners, False arrow-feather. (19)(22) Aronia arbutifolia (L.) Pers., Red chokeberry. (20)(23) Asplenium bradleyi D. C. Eaton, Bradley's spleenwort. -

State of New York City's Plants 2018
STATE OF NEW YORK CITY’S PLANTS 2018 Daniel Atha & Brian Boom © 2018 The New York Botanical Garden All rights reserved ISBN 978-0-89327-955-4 Center for Conservation Strategy The New York Botanical Garden 2900 Southern Boulevard Bronx, NY 10458 All photos NYBG staff Citation: Atha, D. and B. Boom. 2018. State of New York City’s Plants 2018. Center for Conservation Strategy. The New York Botanical Garden, Bronx, NY. 132 pp. STATE OF NEW YORK CITY’S PLANTS 2018 4 EXECUTIVE SUMMARY 6 INTRODUCTION 10 DOCUMENTING THE CITY’S PLANTS 10 The Flora of New York City 11 Rare Species 14 Focus on Specific Area 16 Botanical Spectacle: Summer Snow 18 CITIZEN SCIENCE 20 THREATS TO THE CITY’S PLANTS 24 NEW YORK STATE PROHIBITED AND REGULATED INVASIVE SPECIES FOUND IN NEW YORK CITY 26 LOOKING AHEAD 27 CONTRIBUTORS AND ACKNOWLEGMENTS 30 LITERATURE CITED 31 APPENDIX Checklist of the Spontaneous Vascular Plants of New York City 32 Ferns and Fern Allies 35 Gymnosperms 36 Nymphaeales and Magnoliids 37 Monocots 67 Dicots 3 EXECUTIVE SUMMARY This report, State of New York City’s Plants 2018, is the first rankings of rare, threatened, endangered, and extinct species of what is envisioned by the Center for Conservation Strategy known from New York City, and based on this compilation of The New York Botanical Garden as annual updates thirteen percent of the City’s flora is imperiled or extinct in New summarizing the status of the spontaneous plant species of the York City. five boroughs of New York City. This year’s report deals with the City’s vascular plants (ferns and fern allies, gymnosperms, We have begun the process of assessing conservation status and flowering plants), but in the future it is planned to phase in at the local level for all species. -

Download Authenticated
Ohio Administrative Code Rule 1501:18-1-03 Endangered and threatened species. Effective: January 30, 2021 (A) The following species of plants are designated as endangered in Ohio. (1) Acer pensylvanicum L., Striped maple. (2) Aconitum noveboracense A. Gray, Northern monkshood. (3) Aconitum uncinatum L., Southern monkshood. (4) Adlumia fungosa (Ait.) Greene, Allegheny-vine. (5) Agalinis auriculata (Michx.) Blake, Ear-leaved-foxglove. (6) Agalinis purpurea (L.) Pennell var. parviflora (Benth.) Boivin, Small purple-foxglove. (7) Agalinis skinneriana (Wood) Britt., Skinner's-foxglove. (8) Ageratina aromatica (L.) Spach, Small white snakeroot. (9) Agrostis elliottiana Schultes, Elliott's bent grass. (10) Amelanchier humilis Wiegand, Low serviceberry. (11) Amelanchier interior E.L. Nielsen, Inland serviceberry. (12) Amphidium mougeotii (Bruch, Schimper and W. Gmbel) Schimper, Mougeot's ice moss. (13) Andropogon glomeratus (Walter) Britton, Bushy broom-sedge. Page 1 (14) Androsace occidentalis Pursh, Western rock-jasmine. (15) Anomobryum filiforme (Dicks.) Solms, Common silver moss. (16) Anomodon viticulosus (Hedw.) Hook. and Taylor, Long tail moss. (17) Arabidopsis lyrata (L.) OKane and Al-Shehbaz, Lyre-leaved rock cress. (18) Arabis patens Sullivant, Spreading rock cress. (19) Arctostaphylos uva-ursi (L.) Spreng., Bearberry. (20) Aralia hispida Vent., Bristly sarsaparilla. (21) Arethusa bulbosa L., Dragon's-mouth. (22) Aristida basiramea Engelm. ex Vasey, Forked three-awn grass. (23) Aristida necopina Shinners, False arrow-feather. (24) Aronia arbutifolia (L.) Pers., Red chokeberry. (25) Asplenium bradleyi D.C. Eaton, Bradley's spleenwort. (26) Asplenium resiliens Kunze, Black-stemmed spleenwort. (27) Astragalus neglectus (T. and G.) Sheld., Cooper's milk-vetch. (28) Baptisia australis (L.) R. Br., Blue false indigo. (29) Barbula indica (Hooker) Sprengel in E.G. -

Gori River Basin Substate BSAP
A BIODIVERSITY LOG AND STRATEGY INPUT DOCUMENT FOR THE GORI RIVER BASIN WESTERN HIMALAYA ECOREGION DISTRICT PITHORAGARH, UTTARANCHAL A SUB-STATE PROCESS UNDER THE NATIONAL BIODIVERSITY STRATEGY AND ACTION PLAN INDIA BY FOUNDATION FOR ECOLOGICAL SECURITY MUNSIARI, DISTRICT PITHORAGARH, UTTARANCHAL 2003 SUBMITTED TO THE MINISTRY OF ENVIRONMENT AND FORESTS GOVERNMENT OF INDIA NEW DELHI CONTENTS FOREWORD ............................................................................................................ 4 The authoring institution. ........................................................................................................... 4 The scope. .................................................................................................................................. 5 A DESCRIPTION OF THE AREA ............................................................................... 9 The landscape............................................................................................................................. 9 The People ............................................................................................................................... 10 THE BIODIVERSITY OF THE GORI RIVER BASIN. ................................................ 15 A brief description of the biodiversity values. ......................................................................... 15 Habitat and community representation in flora. .......................................................................... 15 Species richness and life-form -

Abelia X Grandiflora Abeliophyllum Distichum Abies Alba Abies Alba Pendula Abies Balsamea Nana Abies Balsamea Piccolo Abies Ceph
Abelia x grandiflora Abeliophyllum distichum Abies alba Abies alba Pendula Abies balsamea Nana Abies balsamea Piccolo Abies cephalonica Abies concolor Abies concolor Argentea Abies concolor Compacta Abies concolor Piggelmee Abies concolor Violacea Abies fraseri Abies grandis Abies homolepis Abies koreana Abies koreana Bonsai Blue Abies koreana Brevifolia Abies koreana Cis Abies koreana Molli Abies koreana Oberon Abies koreana Piccolo Abies koreana Samling Abies koreana Silberlocke Abies koreana Tundra Abies lasiocarpa Argentea Abies lasiocarpa Compacta Abies nordmanniana Abies nordmanniana Barabits Abies nordmanniana Barabits Giant Abies nordmanniana Emerald Pearl Abies nordmanniana Golden Spreader Abies nordmanniana Pendula Abies pinsapo Glauca Abies pinsapo Kelleris Abies pinsapo var. tazaotana Abies procera Abies procera Glauca Abies procera Rattail Abies sibirica Abies veitchii Abies x arnoldiana Jan Pawel II Abies x insignis Pendula Acaena buchananii Acaena caesiiglauca Frikart Acaena inermis Acaena magellanica Acaena microphylla Acaena microphylla Kupferteppich Acaena microphylla Purpurteppich Acaena novae-zelandiae Acaena pinnatifida Acantholimon glumaceum Acanthus hungaricus Acanthus mollis Acantus spinosus Acer campestre Acer campestre Elsrijk Acer campestre Nanum Acer campestre Queen Elizabeth Acer capillipes Acer freemanii Autumn Blaze Acer griseum Acer japonicum Aconitifolium Acer japonicum Bloodgood Acer japonicum Crimson Queen Acer japonicum Sango-kaku Acer japonicum Vitifolium Acer negundo Aureovariegatum Acer negundo Flamingo -

National List of Vascular Plant Species That Occur in Wetlands 1996
National List of Vascular Plant Species that Occur in Wetlands: 1996 National Summary Indicator by Region and Subregion Scientific Name/ North North Central South Inter- National Subregion Northeast Southeast Central Plains Plains Plains Southwest mountain Northwest California Alaska Caribbean Hawaii Indicator Range Abies amabilis (Dougl. ex Loud.) Dougl. ex Forbes FACU FACU UPL UPL,FACU Abies balsamea (L.) P. Mill. FAC FACW FAC,FACW Abies concolor (Gord. & Glend.) Lindl. ex Hildebr. NI NI NI NI NI UPL UPL Abies fraseri (Pursh) Poir. FACU FACU FACU Abies grandis (Dougl. ex D. Don) Lindl. FACU-* NI FACU-* Abies lasiocarpa (Hook.) Nutt. NI NI FACU+ FACU- FACU FAC UPL UPL,FAC Abies magnifica A. Murr. NI UPL NI FACU UPL,FACU Abildgaardia ovata (Burm. f.) Kral FACW+ FAC+ FAC+,FACW+ Abutilon theophrasti Medik. UPL FACU- FACU- UPL UPL UPL UPL UPL NI NI UPL,FACU- Acacia choriophylla Benth. FAC* FAC* Acacia farnesiana (L.) Willd. FACU NI NI* NI NI FACU Acacia greggii Gray UPL UPL FACU FACU UPL,FACU Acacia macracantha Humb. & Bonpl. ex Willd. NI FAC FAC Acacia minuta ssp. minuta (M.E. Jones) Beauchamp FACU FACU Acaena exigua Gray OBL OBL Acalypha bisetosa Bertol. ex Spreng. FACW FACW Acalypha virginica L. FACU- FACU- FAC- FACU- FACU- FACU* FACU-,FAC- Acalypha virginica var. rhomboidea (Raf.) Cooperrider FACU- FAC- FACU FACU- FACU- FACU* FACU-,FAC- Acanthocereus tetragonus (L.) Humm. FAC* NI NI FAC* Acanthomintha ilicifolia (Gray) Gray FAC* FAC* Acanthus ebracteatus Vahl OBL OBL Acer circinatum Pursh FAC- FAC NI FAC-,FAC Acer glabrum Torr. FAC FAC FAC FACU FACU* FAC FACU FACU*,FAC Acer grandidentatum Nutt. -
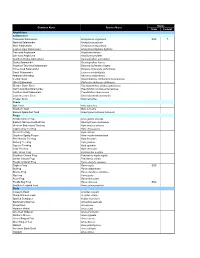
St. Joseph Bay Native Species List
Status Common Name Species Name State Federal Amphibians Salamanders Flatwoods Salamander Ambystoma cingulatum SSC T Marbled Salamander Ambystoma opacum Mole Salamander Ambystoma talpoideum Eastern Tiger Salamander Ambystoma tigrinum tigrinum Two-toed Amphiuma Amphiuma means One-toed Amphiuma Amphiuma pholeter Southern Dusky Salamander Desmognathus auriculatus Dusky Salamander Desmognathus fuscus Southern Two-lined Salamander Eurycea bislineata cirrigera Three-lined Salamander Eurycea longicauda guttolineata Dwarf Salamander Eurycea quadridigitata Alabama Waterdog Necturus alabamensis Central Newt Notophthalmus viridescens louisianensis Slimy Salamander Plethodon glutinosus glutinosus Slender Dwarf Siren Pseudobranchus striatus spheniscus Gulf Coast Mud Salamander Pseudotriton montanus flavissimus Southern Red Salamander Pseudotriton ruber vioscai Eastern Lesser Siren Siren intermedia intermedia Greater Siren Siren lacertina Toads Oak Toad Bufo quercicus Southern Toad Bufo terrestris Eastern Spadefoot Toad Scaphiopus holbrooki holbrooki Frogs Florida Cricket Frog Acris gryllus dorsalis Eastern Narrow-mouthed Frog Gastrophryne carolinensis Western Bird-voiced Treefrog Hyla avivoca avivoca Cope's Gray Treefrog Hyla chrysoscelis Green Treefrog Hyla cinerea Southern Spring Peeper Hyla crucifer bartramiana Pine Woods Treefrog Hyla femoralis Barking Treefrog Hyla gratiosa Squirrel Treefrog Hyla squirella Gray Treefrog Hyla versicolor Little Grass Frog Limnaoedus ocularis Southern Chorus Frog Pseudacris nigrita nigrita Ornate Chorus Frog Pseudacris -

FINAL REPORT PSRA Vegetation Monitoring 2005-2006 PC P502173
Rare Plants and Their Locations at Picayune Strand Restoration Area: Task 4a FINAL REPORT PSRA Vegetation Monitoring 2005-2006 PC P502173 Steven W. Woodmansee and Michael J. Barry [email protected] December 20, 2006 Submitted by The Institute for Regional Conservation 22601 S.W. 152 Avenue, Miami, Florida 33170 George D. Gann, Executive Director Submitted to Mike Duever, Ph.D. Senior Environmental Scientist South Florida Water Management District Fort Myers Service Center 2301 McGregor Blvd. Fort Myers, Florida 33901 Table of Contents Introduction 03 Methods 03 Results and Discussion 05 Acknowledgements 38 Citations 39 Tables: Table 1: Rare plants recorded in the vicinity of the Vegetation Monitoring Transects 05 Table 2: The Vascular Plants of Picayune Strand State Forest 24 Figures: Figure 1: Picayune Strand Restoration Area 04 Figure 2: PSRA Rare Plants: Florida Panther NWR East 13 Figure 3: PSRA Rare Plants: Florida Panther NWR West 14 Figure 4: PSRA Rare Plants: PSSF Northeast 15 Figure 5: PSRA Rare Plants: PSSF Northwest 16 Figure 6: PSRA Rare Plants: FSPSP West 17 Figure 7: PSRA Rare Plants: PSSF Southeast 18 Figure 8: PSRA Rare Plants: PSSF Southwest 19 Figure 9: PSRA Rare Plants: FSPSP East 20 Figure 10: PSRA Rare Plants: TTINWR 21 Cover Photo: Bulbous adder’s tongue (Ophioglossum crotalophoroides), a species newly recorded for Collier County, and ranked as Critically Imperiled in South Florida by The Institute for Regional Conservation taken by the primary author. 2 Introduction The South Florida Water Management District (SFWMD) plans on restoring the hydrology at Picayune Strand Restoration Area (PSRA) see Figure 1. -

Chemical and Biological Research on Herbal Medicines Rich in Xanthones
molecules Review Chemical and Biological Research on Herbal Medicines Rich in Xanthones Jingya Ruan 1, Chang Zheng 1, Yanxia Liu 1, Lu Qu 2, Haiyang Yu 2, Lifeng Han 2, Yi Zhang 1,2,* and Tao Wang 1,2,* 1 Tianjin State Key Laboratory of Modern Chinese Medicine, 312 Anshanxi Road, Nankai District, Tianjin 300193, China; [email protected] (J.R.); [email protected] (C.Z.); [email protected] (Y.L.) 2 Tianjin Key Laboratory of TCM Chemistry and Analysis, Institute of Traditional Chinese Medicine, Tianjin University of Traditional Chinese Medicine, 312 Anshan Road, Nankai District, Tianjin 300193, China; [email protected] (L.Q.); [email protected] (H.Y.); [email protected] (L.H.) * Correspondence: [email protected] (Y.Z.); [email protected] (T.W.); Tel./Fax: +86-22-5959-6163 (Y.Z. & T.W.) Received: 11 September 2017; Accepted: 9 October 2017; Published: 11 October 2017 Abstract: Xanthones, as some of the most active components and widely distributed in various herb medicines, have drawn more and more attention in recent years. So far, 168 species of herbal plants belong to 58 genera, 24 families have been reported to contain xanthones. Among them, Calophyllum, Cratoxylum, Cudrania, Garcinia, Gentiana, Hypericum and Swertia genera are plant resources with great development prospect. This paper summarizes the plant resources, bioactivity and the structure-activity relationships (SARs) of xanthones from references published over the last few decades, which may be useful for new drug research and development on xanthones. Keywords: herbal medicines; xanthones; plant sources; pharmacology; gambogic acid; structure-activity relationships 1. Introdution Xanthones (IUPAC name 9H-xanthen-9-one) are a kind of phenolic acid with a three-ring skeleton, widely distributed in herbal medicines. -

Download Download
Natural Barrens and Post Oak Flatwoods in Posey and Spencer Counties, Indiana James R. Aldrich and Michael A. Homoya Indiana Natural Heritage Program, Indiana Department of Natural Resources Indianapolis, Indiana 46204 Introduction Post oak flatwoods are xeric forested communities that are dominated by Quercus stellata with a relatively open canopy that allows a great deal of dispersed light to reach the forest floor. This forested community lacks the typical shrub-layer found in more mesic forested communities and appears "savanna-like". Characteristically, post oak flatwoods in Indiana are found on poorly drained nearly level soils of alluvial lacustrine terraces of the Ohio River and other major streams in the unglaciated region of the Wabash Lowland Physiographic Province (12). The understory is dominated by sedges and the content of organic matter in the soil appears to be very low. The barrens described herein are relatively small natural openings surrounded by post oak flatwoods where a fragipan is at or very near the surface. The vegetation in these bar- rens is not dominated by prairie grasses and forbs, but is closely related to the vegeta- tion of sandstone glades described for Illinois (21) and Missouri (18). Contemporary southern "flatwoods" of the Illinois Tillplain dominated by sweetgum {Liquidambar styraciflua), beech (Fagus grandifolia) and red maple (Acer rubrum) in southwestern Ohio (4) and southeastern Indiana have been described in detail (9,13,15,17). Indiana "barrens" dominated by prairie forbs and grasses (7) have been discovered recently and discussed (3,10). Flatwoods dominated by post oak have received limited attention in Illinois (16) where they have been referred to as "southern flatwoods" (21) and very little has been written about the Indiana post oak flatwoods.