A 1 Phylogenetic Validity Test Using Morphostructural Conspicuity 2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

What Really Hampers Taxonomy and Conservation? a Riposte to Garnett and Christidis (2017)
Zootaxa 4317 (1): 179–184 ISSN 1175-5326 (print edition) http://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2017 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4317.1.10 http://zoobank.org/urn:lsid:zoobank.org:pub:88FA0944-D3CF-4A7D-B8FB-BAA6A3A76744 What really hampers taxonomy and conservation? A riposte to Garnett and Christidis (2017) MARCOS A. RAPOSO1,2, RENATA STOPIGLIA3,4, GUILHERME RENZO R. BRITO1,5, FLÁVIO A. BOCKMANN3,6, GUY M. KIRWAN1,7, JEAN GAYON2 & ALAIN DUBOIS4 1 Setor de Ornitologia, Departamento de Vertebrados, Museu Nacional, Universidade Federal do Rio de Janeiro, Quinta da Boa Vista, s/n, 20940–040 Rio de Janeiro, RJ, Brazil. [email protected] (MAR), [email protected] (GRRB), [email protected] (GMK) 2 UMR 8590, IHPST–Institut d'Histoire et de Philosophie des Sciences et des Techniques, UMR 8590, Université Paris 1 Panthéon- Sorbonne & CNRS, 13 rue du Four, 75006 Paris, France. [email protected] (JG) 3 Laboratório de Ictiologia de Ribeirão Preto, Departamento de Biologia, FFCLRP, Universidade de São Paulo, Av. dos Bandeirantes 3900, 14040–901 Ribeirão Preto, SP, Brazil. [email protected] (RS), [email protected] (FAB) 4 Institut de Systématique, Évolution, Biodiversité, ISYEB – UMR 7205 – CNRS, MNHN, UPMC, EPHE, Muséum national d’Histoire naturelle, Sorbonne Universités, 25 rue Cuvier, CP 30, 75005, Paris, France. [email protected] (AD). 5 Departamento de Zoologia, Instituto de Biologia, Universidade Federal do Rio de Janeiro, Av. Carlos Chagas Filho, 373, 21941- 902, Rio de Janeiro, RJ, Brazil. 6 Programa de Pós-Graduação em Biologia Comparada, FFCLRP, Universidade de São Paulo, Av. -

Cop18 Prop. 39
Original language: English CoP18 Prop. 39 CONVENTION ON INTERNATIONAL TRADE IN ENDANGERED SPECIES OF WILD FAUNA AND FLORA ____________________ Eighteenth meeting of the Conference of the Parties Colombo (Sri Lanka), 23 May – 3 June 2019 CONSIDERATION OF PROPOSALS FOR AMENDMENT OF APPENDICES I AND II A. Proposal Inclusion of Echinotriton chinhaiensis (Chang, 1932) and Echinotriton maxiquadratus Hou, Wu, Yang, Zheng, Yuan, and Li, 2014, both of which are endemic to China in Appendix Ⅱ, in accordance with Article Ⅱ, paragraph 2 (a) of the Convention and satisfying Criterion B in Annex 2a of Resolution Conf. 9.24 (Rev. CoP17). The international trade of these two newts should be monitored to minimise the impact of illegal hunting driven by international pet trade or collection on the survival of these two critically endangered species B. Proponent China*: C. Supporting statement 1. Taxonomy 1.1 Class: Amphibia 1.2 Order: Caudata 1.3 Family: Salamandridae 1.4 Genus, species or subspecies, including author and year: 1.5 Scientific synonyms: Echinotriton chinhaiensis: Tylototriton chinhaiensis Chang, 1932; Tylototriton (Echinotriton) chinhaiensis; Pleurodeles chinhaiensis (Chang, 1932); Pleurodeles (Tylototrion) chinhaiensis 1.6 Common names: English: E. chinhaiensis: Chinhai Spiny Newt, Chinhai Spiny Crocodile Newt E. maxiquadratus: Mountain Spiny Newt, Mountain Spiny Crocodile Newt French: Spanish: 1.7 Code numbers: N/A * The geographical designations employed in this document do not imply the expression of any opinion whatsoever on the part of the CITES Secretariat (or the United Nations Environment Programme) concerning the legal status of any country, territory, or area, or concerning the delimitation of its frontiers or boundaries. The responsibility for the contents of the document rests exclusively with its author. -

Notophthalmus Perstriatus) Version 1.0
Species Status Assessment for the Striped Newt (Notophthalmus perstriatus) Version 1.0 Striped newt eft. Photo credit Ryan Means (used with permission). May 2018 U.S. Fish and Wildlife Service Region 4 Jacksonville, Florida 1 Acknowledgements This document was prepared by the U.S. Fish and Wildlife Service’s North Florida Field Office with assistance from the Georgia Field Office, and the striped newt Species Status Assessment Team (Sabrina West (USFWS-Region 8), Kaye London (USFWS-Region 4) Christopher Coppola (USFWS-Region 4), and Lourdes Mena (USFWS-Region 4)). Additionally, valuable peer reviews of a draft of this document were provided by Lora Smith (Jones Ecological Research Center) , Dirk Stevenson (Altamaha Consulting), Dr. Eric Hoffman (University of Central Florida), Dr. Susan Walls (USGS), and other partners, including members of the Striped Newt Working Group. We appreciate their comments, which resulted in a more robust status assessment and final report. EXECUTIVE SUMMARY This Species Status Assessment (SSA) is an in-depth review of the striped newt's (Notophthalmus perstriatus) biology and threats, an evaluation of its biological status, and an assessment of the resources and conditions needed to maintain species viability. We begin the SSA with an understanding of the species’ unique life history, and from that we evaluate the biological requirements of individuals, populations, and species using the principles of population resiliency, species redundancy, and species representation. All three concepts (or analogous ones) apply at both the population and species levels, and are explained that way below for simplicity and clarity as we introduce them. The striped newt is a small salamander that uses ephemeral wetlands and the upland habitat (scrub, mesic flatwoods, and sandhills) that surrounds those wetlands. -

Zootaxa, a New Species of Paramesotriton (Caudata
Zootaxa 1775: 51–60 (2008) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA Copyright © 2008 · Magnolia Press ISSN 1175-5334 (online edition) A new species of Paramesotriton (Caudata: Salamandridae) from Guizhou Province, China HAITAO ZHAO1, 2, 5, JING CHE2,5, WEIWEI ZHOU2, YONGXIANG CHEN1, HAIPENG ZHAO3 & YA-PING ZHANG2 ,4 1Department of Environment and Life Science, Bijie College, Guizhou 551700, China 2State Key Laboratory of Genetic Resources and Evolution, Kunming Institute of Zoology, the Chinese Academy of Sciences, Kunming 650223, China 3School of Life Science, Southwest University, Chongqing 400715, China 4Corresponding authors. E-mail: [email protected] 5 These authors contributed equally to this work. Abstract We describe a new species of salamander, Paramesotriton zhijinensis, from Guizhou Province, China. The generic allo- cation of the new species is based on morphological and molecular characters. In morphology, it is most similar to Paramesotriton chinensis but differs in having distinct gland emitting a malodorous secretion (here named scent gland), a postocular stripe, and two non-continuous, dorsolateral stripes on the dorsolateral ridges. Furthermore, neoteny was observed in most individuals of the new species. This has not been previously reported to occur in any other species of Paramesotriton. Analysis of our molecular data suggests that this species a third major evolutionary lineage in the genus Paramesotriton. Key words: Caudata; Salamandridae; Paramesotriton zhijinensis; new species; scent gland; Guizhou; China Introduction Guizhou Province, located in the southwestern mountainous region of China, is known for its rich amphibian faunal diversity (Liu and Hu 1961). During recent surveys of the Guizhou herpetofauna (July, September, and November, 2006; January and September, 2007), we collected salamanders superficially resembling Parame- sotriton chinensis (Gray). -

Summary Report of Freshwater Nonindigenous Aquatic Species in U.S
Summary Report of Freshwater Nonindigenous Aquatic Species in U.S. Fish and Wildlife Service Region 4—An Update April 2013 Prepared by: Pam L. Fuller, Amy J. Benson, and Matthew J. Cannister U.S. Geological Survey Southeast Ecological Science Center Gainesville, Florida Prepared for: U.S. Fish and Wildlife Service Southeast Region Atlanta, Georgia Cover Photos: Silver Carp, Hypophthalmichthys molitrix – Auburn University Giant Applesnail, Pomacea maculata – David Knott Straightedge Crayfish, Procambarus hayi – U.S. Forest Service i Table of Contents Table of Contents ...................................................................................................................................... ii List of Figures ............................................................................................................................................ v List of Tables ............................................................................................................................................ vi INTRODUCTION ............................................................................................................................................. 1 Overview of Region 4 Introductions Since 2000 ....................................................................................... 1 Format of Species Accounts ...................................................................................................................... 2 Explanation of Maps ................................................................................................................................ -

Conservation Matters: CITES and New Herp Listings
Conservation matters:FEATURE | CITES CITES and new herp listings The red-tailed knobby newt (Tylototriton kweichowensis) now has a higher level of protection under CITES. Photo courtesy Milan Zygmunt/www. shutterstock.com What are the recent CITES listing changes and what do they mean for herp owners? Dr. Thomas E.J. Leuteritz from the U.S. Fish & Wildlife Service explains. id you know that your pet It is not just live herp may be a species of animals that are protected wildlife? Many covered by CITES, exotic reptiles and but parts and Damphibians are protected under derivatives too, such as crocodile skins CITES, also known as the Convention that feature in the on International Trade in Endangered leather trade. Plants Species of Wild Fauna and Flora. and timber are also Initiated in 1973, CITES is an included. international agreement currently Photo courtesy asharkyu/ signed by 182 countries and the www.shutterstock.com European Union (also known as responsibility of the Secretary of the How does CITES work? Parties), which regulates Interior, who has tasked the U.S. Fish Species protected by CITES are international trade in more than and Wildlife Service (USFWS) as the included in one of three lists, 35,000 wild animal and plant species, lead agency responsible for the referred to as Appendices, according including their parts, products, and Convention’s implementation. You to the degree of protection they derivatives. can help USFWS conserve these need: Appendix I includes species The aim of CITES is to ensure that species by complying with CITES threatened with extinction and international trade in specimens of and other wildlife laws to ensure provides the greatest level of wild animals and plants does not that your activities as a pet owner or protection, including restrictions on threaten their survival in the wild. -

Variations in Tetrodotoxin Levels in Populations of Taricha Granulosa
www.nature.com/scientificreports OPEN Variations in tetrodotoxin levels in populations of Taricha granulosa are expressed in the morphology of their cutaneous glands Pedro Luiz Mailho-Fontana1*, Carlos Jared1, Marta Maria Antoniazzi1, Juliana Mozer Sciani 2, Daniel Carvalho Pimenta 1, Amber N. Stokes3, Taran Grant4, Edmund D. Brodie III5 & Edmund D. Brodie Jr.6 Tetrodotoxin (TTX), one of the most toxic substances in nature, is present in bacteria, invertebrates, fshes, and amphibians. Marine organisms seem to bioaccumulate TTX from their food or acquire it from symbiotic bacteria, but its origin in amphibians is unclear. Taricha granulosa can exhibit high TTX levels, presumably concentrated in skin poison glands, acting as an agent of selection upon predatory garter snakes (Thamnophis). This co-evolutionary arms race induces variation in T. granulosa TTX levels, from very high to undetectable. Using morphology and biochemistry, we investigated diferences in toxin localization and quality between two populations at the extremes of toxicity. TTX concentration within poison glands is related to the volume of a single cell type in which TTX occurs exclusively in distinctive secretory granules, suggesting a relationship between granule structure and chemical composition. TTX was detected in mucous glands in both populations, contradicting the general understanding that these glands do not secrete defensive chemicals and expanding currently held interpretations of amphibian skin gland functionality. Skin secretions of the two populations difered in low-mass molecules and proteins. Our results demonstrate that interpopulation variation in TTX levels is related to poison gland morphology. Tetrodotoxin (TTX) is one of the most toxic and well-studied but still mysterious natural products. -

Calotriton Asper)
Amphibia-Reptilia (2014) DOI:10.1163/15685381-00002921 Life history trait differences between a lake and a stream-dwelling population of the Pyrenean brook newt (Calotriton asper) Neus Oromi1,∗, Fèlix Amat2, Delfi Sanuy1, Salvador Carranza3 Abstract. The Pyrenean brook newt (Calotriton asper) is a salamandrid that mostly lives in fast running and cold mountain- streams, although some populations are also found in lakes. In the present work, we report in detail on the occurrence of facultative paedomorphosis traits in a population from a Pyrenean high altitude lake. We compare its morphology, life history traits and mitochondrial DNA variation with a nearby lotic metamorphic population. Our results indicate that the lacustrine newts are smaller and present a less developed sexual dimorphism, smooth skin, and that 53% of the adults retain gills at different degrees of development, but not gill slits. Although both populations and sexes have the same age at sexual maturity (four years), the lacustrine population presents higher longevity (12 and 9 years for males and females, respectively) than the one living in the stream (8 and 9 years). The variation on the climatic conditions at altitudinal scale is probably the main cause of the differences in life history traits found between the two populations. The food availability, which could to be limiting in the lacustrine population, is another factor that can potentially affect body size. These results are congruent with the significant mitochondrial DNA genetic isolation between populations, probably a consequence of the lack of juvenile dispersal. We found low cytochrome b variability and significant genetic structuring in the lake population that is very remarkably considering the short distance to the nearby stream population and the whole species’ pattern. -

Zootaxa, a New Species in the Tylototriton Asperrimus Group
TERMS OF USE This pdf is provided by Magnolia Press for private/research use. Commercial sale or deposition in a public library or website is prohibited. Zootaxa 2650: 19–32 (2010) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2010 · Magnolia Press ISSN 1175-5334 (online edition) A new species in the Tylototriton asperrimus group (Caudata: Salamandridae) from central Laos BRYAN L. STUART1,2,6, SOMPHOUTHONE PHIMMACHAK3,4, NIANE SIVONGXAY3 & WILLIAM G. ROBICHAUD5 1North Carolina Museum of Natural Sciences, 11 West Jones Street, Raleigh NC 27601, USA 2Museum of Vertebrate Zoology, 3101 Valley Life Sciences Building, University of California, Berkeley CA 94720-3160, USA 3National University of Laos, Faculty of Sciences, Vientiane, Lao PDR 4Wildlife Conservation Society, P.O. Box 6712, Vientiane, Lao PDR 5Nam Theun 2 Watershed Management and Protection Authority, P.O. Box 190, Thakhek, Khammouan Province, Lao PDR 6Corresponding author. E-mail: [email protected] Abstract A new species in the morphologically conservative Tylototriton asperrimus group is described from Khammouan Province, Laos. Molecular phylogenetic analysis of mitochondrial DNA confirms its placement in the T. asperrimus group. Tylototriton notialis sp. nov. is diagnosable in mitochondrial DNA, nuclear DNA, and morphology from its congeners. The new species represents the first record of the genus from Laos, and is the southernmost known member of the T. asperrimus group. Key words: Caudata, Laos, Southeast Asia, Tylototriton Introduction The Asian newt genus Tylototriton Anderson, 1871 contains eight species (Dubois & Raffaëlli 2009) distributed from Nepal to northern Vietnam. These eight species consist of two clades, the T. verrucosus group (= subgenus Tylototriton Dubois & Raffaëlli 2009) containing T. -

A Review of Chemical Defense in Poison Frogs (Dendrobatidae): Ecology, Pharmacokinetics, and Autoresistance
Chapter 21 A Review of Chemical Defense in Poison Frogs (Dendrobatidae): Ecology, Pharmacokinetics, and Autoresistance Juan C. Santos , Rebecca D. Tarvin , and Lauren A. O’Connell 21.1 Introduction Chemical defense has evolved multiple times in nearly every major group of life, from snakes and insects to bacteria and plants (Mebs 2002 ). However, among land vertebrates, chemical defenses are restricted to a few monophyletic groups (i.e., clades). Most of these are amphibians and snakes, but a few rare origins (e.g., Pitohui birds) have stimulated research on acquired chemical defenses (Dumbacher et al. 1992 ). Selective pressures that lead to defense are usually associated with an organ- ism’s limited ability to escape predation or conspicuous behaviors and phenotypes that increase detectability by predators (e.g., diurnality or mating calls) (Speed and Ruxton 2005 ). Defended organisms frequently evolve warning signals to advertise their defense, a phenomenon known as aposematism (Mappes et al. 2005 ). Warning signals such as conspicuous coloration unambiguously inform predators that there will be a substantial cost if they proceed with attack or consumption of the defended prey (Mappes et al. 2005 ). However, aposematism is likely more complex than the simple pairing of signal and defense, encompassing a series of traits (i.e., the apose- matic syndrome) that alter morphology, physiology, and behavior (Mappes and J. C. Santos (*) Department of Zoology, Biodiversity Research Centre , University of British Columbia , #4200-6270 University Blvd , Vancouver , BC , Canada , V6T 1Z4 e-mail: [email protected] R. D. Tarvin University of Texas at Austin , 2415 Speedway Stop C0990 , Austin , TX 78712 , USA e-mail: [email protected] L. -

50 CFR Ch. I (10–1–20 Edition) § 16.14
§ 15.41 50 CFR Ch. I (10–1–20 Edition) Species Common name Serinus canaria ............................................................. Common Canary. 1 Note: Permits are still required for this species under part 17 of this chapter. (b) Non-captive-bred species. The list 16.14 Importation of live or dead amphib- in this paragraph includes species of ians or their eggs. non-captive-bred exotic birds and coun- 16.15 Importation of live reptiles or their tries for which importation into the eggs. United States is not prohibited by sec- Subpart C—Permits tion 15.11. The species are grouped tax- onomically by order, and may only be 16.22 Injurious wildlife permits. imported from the approved country, except as provided under a permit Subpart D—Additional Exemptions issued pursuant to subpart C of this 16.32 Importation by Federal agencies. part. 16.33 Importation of natural-history speci- [59 FR 62262, Dec. 2, 1994, as amended at 61 mens. FR 2093, Jan. 24, 1996; 82 FR 16540, Apr. 5, AUTHORITY: 18 U.S.C. 42. 2017] SOURCE: 39 FR 1169, Jan. 4, 1974, unless oth- erwise noted. Subpart E—Qualifying Facilities Breeding Exotic Birds in Captivity Subpart A—Introduction § 15.41 Criteria for including facilities as qualifying for imports. [Re- § 16.1 Purpose of regulations. served] The regulations contained in this part implement the Lacey Act (18 § 15.42 List of foreign qualifying breed- U.S.C. 42). ing facilities. [Reserved] § 16.2 Scope of regulations. Subpart F—List of Prohibited Spe- The provisions of this part are in ad- cies Not Listed in the Appen- dition to, and are not in lieu of, other dices to the Convention regulations of this subchapter B which may require a permit or prescribe addi- § 15.51 Criteria for including species tional restrictions or conditions for the and countries in the prohibited list. -
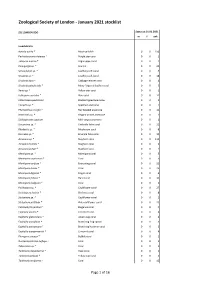
Jan 2021 ZSL Stocklist.Pdf (699.26
Zoological Society of London - January 2021 stocklist ZSL LONDON ZOO Status at 01.01.2021 m f unk Invertebrata Aurelia aurita * Moon jellyfish 0 0 150 Pachyclavularia violacea * Purple star coral 0 0 1 Tubipora musica * Organ-pipe coral 0 0 2 Pinnigorgia sp. * Sea fan 0 0 20 Sarcophyton sp. * Leathery soft coral 0 0 5 Sinularia sp. * Leathery soft coral 0 0 18 Sinularia dura * Cabbage leather coral 0 0 4 Sinularia polydactyla * Many-fingered leather coral 0 0 3 Xenia sp. * Yellow star coral 0 0 1 Heliopora coerulea * Blue coral 0 0 12 Entacmaea quadricolor Bladdertipped anemone 0 0 1 Epicystis sp. * Speckled anemone 0 0 1 Phymanthus crucifer * Red beaded anemone 0 0 11 Heteractis sp. * Elegant armed anemone 0 0 1 Stichodactyla tapetum Mini carpet anemone 0 0 1 Discosoma sp. * Umbrella false coral 0 0 21 Rhodactis sp. * Mushroom coral 0 0 8 Ricordea sp. * Emerald false coral 0 0 19 Acropora sp. * Staghorn coral 0 0 115 Acropora humilis * Staghorn coral 0 0 1 Acropora yongei * Staghorn coral 0 0 2 Montipora sp. * Montipora coral 0 0 5 Montipora capricornis * Coral 0 0 5 Montipora confusa * Encrusting coral 0 0 22 Montipora danae * Coral 0 0 23 Montipora digitata * Finger coral 0 0 6 Montipora foliosa * Hard coral 0 0 10 Montipora hodgsoni * Coral 0 0 2 Pocillopora sp. * Cauliflower coral 0 0 27 Seriatopora hystrix * Bird nest coral 0 0 8 Stylophora sp. * Cauliflower coral 0 0 1 Stylophora pistillata * Pink cauliflower coral 0 0 23 Catalaphyllia jardinei * Elegance coral 0 0 4 Euphyllia ancora * Crescent coral 0 0 4 Euphyllia glabrescens * Joker's cap coral 0 0 2 Euphyllia paradivisa * Branching frog spawn 0 0 3 Euphyllia paraancora * Branching hammer coral 0 0 3 Euphyllia yaeyamaensis * Crescent coral 0 0 4 Plerogyra sinuosa * Bubble coral 0 0 1 Duncanopsammia axifuga + Coral 0 0 2 Tubastraea sp.