Optic Neuritis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Eyelid and Orbital Infections
27 Eyelid and Orbital Infections Ayub Hakim Department of Ophthalmology, Western Galilee - Nahariya Medical Center, Nahariya, Israel 1. Introduction The major infections of the ocular adnexal and orbital tissues are preseptal cellulitis and orbital cellulitis. They occur more frequently in children than in adults. In Schramm's series of 303 cases of orbital cellulitis, 68% of the patients were younger than 9 years old and only 17% were older than 15 years old. Orbital cellulitis is less common, but more serious than preseptal. Both conditions happen more commonly in the winter months when the incidence of paranasal sinus infections is increased. There are specific causes for each of these types of cellulitis, and each may be associated with serious complications, including vision loss, intracranial infection and death. Studies of orbital cellulitis and its complication report mortality in 1- 2% and vision loss in 3-11%. In contrast, mortality and vision loss are extremely rare in preseptal cellulitis. 1.1 Definitions Preseptal and orbital cellulites are the most common causes of acute orbital inflammation. Preseptal cellulitis is an infection of the soft tissue of the eyelids and periocular region that is localized anterior to the orbital septum outside the bony orbit. Orbital cellulitis ( 3.5 per 100,00 ) is an infection of the soft tissues of the orbit that is localized posterior to the orbital septum and involves the fat and muscles contained within the bony orbit. Both types are normally distinguished clinically by anatomic location. 1.2 Pathophysiology The soft tissues of the eyelids, adnexa and orbit are sterile. Infection usually originates from adjacent non-sterile sites but may also expand hematogenously from distant infected sites when septicemia occurs. -

Department of Ophthalmology Medical Faculty of Padjadjaran University Cicendo Eye Hospital, the National Eye Center Bandung
1 DEPARTMENT OF OPHTHALMOLOGY MEDICAL FACULTY OF PADJADJARAN UNIVERSITY CICENDO EYE HOSPITAL, THE NATIONAL EYE CENTER BANDUNG Case report : Clinical features and Diagnosis of Neuromyelitis Optica Spectrum Disorder (NMOSD) Presenter : Lucy Nofrida Siburian Supervisor : DR. Bambang Setiohaji, dr., SpM(K)., MH.Kes Has been reviewed and approved by supervisor of neuro-ophthalmology unit DR. Bambang Setiohaji, dr., SpM(K)., MH.Kes Friday, August 04, 2017 07.00 am 2 Abstract Introduction : Neuromyelitis optica spectrum disorder (NMOSD), previously known as Devic’s disease, is an inflammatory CNS syndrome distinct from multiple sclerosis (MS). It is characterized by severe, immune-mediated demyelination and axonal damage predominantly targeting the optic nerves and spinal cord though rarely the brain is also involved. Most patients with NMO and many with NMOSD have autoantibodies against the water channel aquaporin-4(AQP4-Ab), which are thought to be pathogenic. However, some patients are seronegative for AQP4-Abs and the lack of a biomarker makes diagnosis and management of these patients difficult. Aim : To present an NMO case and to know the current diagnosis criteria of NMOSD Case report : A woman, 42 years old, came to neuro-ophthalmology unit of Cicendo eye hospital on March 14, 2017 with sudden blurred vision on the right eye (RE) two days before admission without eye movement pain. Physical examination and body weight were normal. Visual acuity (VA) of the right eye (RE) was 1/300 and the best corrected VA on the left eye was 1.0. Anterior segment on the RE showed relative afferent pupillary defect grade 3 (RAPD), others were normal and so is on the LE. -

A Case of Anterior Ischemic Optic Neuropathy Associated with Uveitis
Clinical Ophthalmology Dovepress open access to scientific and medical research Open Access Full Text Article CASE REPORT A case of anterior ischemic optic neuropathy associated with uveitis Michitaka Sugahara Introduction: Here, we describe a patient who presented with anterior ischemic optic Takayuki Fujimoto neuropathy (AION) and subsequently developed uveitis. Kyoko Shidara Case: A 69-year-old man was referred to our hospital and initially presented with best-corrected Kenji Inoue visual acuities (BCVA) of 20/40 (right eye) and 20/1000 (left eye) and relative afferent pupillary Masato Wakakura defect. Slit-lamp examination revealed no signs of ocular inflammation in either eye. Fundus examination revealed left-eye swelling and a pale superior optic disc, and Goldmann perimetry Inouye Eye Hospital, Tokyo, Japan revealed left-eye inferior hemianopia. The patient was diagnosed with nonarteritic AION in the left eye. One week later, the patient returned to the hospital because of vision loss. The BCVA of the left eye was so poor that the patient could only count fingers. Slit-lamp examination revealed 1+ cells in the anterior chamber and the anterior vitreous in both eyes. Funduscopic examination revealed vasculitis and exudates in both eyes. The patient was diagnosed with bilateral panuveitis, and treatment with topical betamethasone was started. No other physical findings resulting from other autoimmune or infectious diseases were found. No additional treatments were administered, and optic disc edema in the left eye improved, and the retinal exudates disappeared in 3 months. The patient’s BCVA improved after cataract surgery was performed. Conclusion: Panuveitis most likely manifests after the development of AION. -

Contrast Sensitivity Function in Graves' Ophthalmopathy and Dysthyroid Optic Neuropathy Br J Ophthalmol: First Published As 10.1136/Bjo.77.11.709 on 1 November 1993
Britishjournal ofOphthalmology 1993; 77: 709-712 709 Contrast sensitivity function in Graves' ophthalmopathy and dysthyroid optic neuropathy Br J Ophthalmol: first published as 10.1136/bjo.77.11.709 on 1 November 1993. Downloaded from Maria S A Suttorp-Schulten, Rob Tijssen, Maarten Ph Mourits, Patricia Apkarian Abstract defocus greatly facilitates the process of subjec- Contrast sensitivity function was measured by tive refraction correction, but reduced contrast a computer automated method on 38 eyes with sensitivity at low spatial frequencies may present dysthyroid optic neuropathy and 34 eyes with with normal Snellen acuity. As there are various Graves' ophthalmopathy only. The results degrees ofvisual loss within the group ofpatients were compared with 74 healthy control eyes. with dysthyroid neuropathy, assessment of Disturbances of contrast sensitivity functions spatial vision across the frequency and contrast were found in both groups when compared with spectrum may reveal visual impairment not controls. The eyes affected with dysthyroid readily detected by standard visual acuity optic neuropathy showed pronounced loss of measures. contrast sensitivity in the low frequency range, The contrast sensitivity function has proved a which facilitates differentiation between the useful tool for detecting visual disturbances two groups. when Snellen acuity fails to show comparable (BrJ Ophthalmol 1993; 77: 709-712) dysfunction - for example, in glaucoma,'4 retinal disease,'516 and pterygia." The clinical potential for contrast sensitivity functions has also been Graves' ophthalmopathy is related to thyroid demonstrated in patients with optic neuro- disease and is characterised by oedema and pathies, " 2"02' including dysthyroid optic neuro- infiltration ofthe extraocular muscles and orbital pathy."22 This study compares the contrast tissue. -

Ocular Dysmetria in a Patient with Charcot-‐Marie-‐ Tooth Disease
Ocular Dysmetria in a Patient with Charcot-Marie- Tooth Disease Michelle Lee, OD A patient with the inherited neuropathy, Charcot-Marie-Tooth disease (CMT), presents with ocular dysmetria. Although abnormal ocular motility has not been reported in CMT patients, the absence of other etiologies indicates a possible ocular manifestation. CASE HISTORY • Patient demographics: 74 year old Caucasian male • Chief complaint: no visual or ocular complaints • Ocular History o Mild cataracts OU o Dry eye syndrome OU o Refractive error OU • Medical history o Charcot-Marie-Tooth disease o Asthma o Hypercholesterolemia o Herpes zoster o Chronic lower bacK pain o Dermatitis o Obstructive sleep apnea • Medications o Albuterol o Gabapentin o Meloxicam o Mometasone furoate o Oxybutynin chloride o Simvastatin o Tamusolisn HCL o Aspirin o Vitamin D • Ocular medications: artificial tears prn OU • Family history: father and grandfather also with CMT PERTINENT FINDINGS • Clinical o Mixed hypometric and hypermetric saccades with intermittent disconjugate movement o Trace restriction of lateral gaze and inferior temporal OS o Ptosis OD o Borderline reduced contrast sensitivity OD, mildly reduced contrast sensitivity OS o Pertinent negatives: no evidence of light-near-dissociation, no signs of optic neuropathy 1 of 4 • Physical o Abnormal gait • Lab studies o EMG consistent with positive family history of CMT • Radiology studies o MRI (04/13): no intracranial mass or acute infarcts seen, no evidence of cerebellar abnormality noted DIFFERENTIAL DIAGNOSIS • Primary/leading -

Postoperative Eye Protection After Cataract Surgery Anterior Uveitis Responds to Ganciclovir, but the Relapse Rate Is High and Prolonged Therapy May Be Required
Correspondence 1152 Sir, 4 Ioannidis AS, Bacon J, Frith P. Juxtapapillary cytomegalovirus Cytomegalovirus and Eye retinitis with optic neuritis. J Neuroophthalmol 2008; 28(2): 128–130. 5 Mansour AM. Cytomegalovirus optic neuritis. Curr Opin We read with interest the very comprehensive article Ophthalmol 1997; 8(3): 55–58. by Carmichael on cytomegalovirus (CMV) and eye.1 6 Patil AJ, Sharma A, Kenney MC, Kuppermann BD. In addition to the clinical features reported by the Valganciclovir in the treatment of cytomegalovirus retinitis author,1 we would like to highlight some additional in HIV-infected patients. Clin Ophthalmol 2012; 4: 111–119. salient clinical points associated with CMV and eye. With regard to clinical manifestation of CMV anterior R Agrawal uveitis, the iris atrophy is patchy or diffuse, with no posterior synechiae and no posterior segment changes.2 Department of Ophthalmology, Tan Tock Seng It is usually associated with increased intraocular Hospital, Singapore pressure.2 Chee and Jap3 also reported the presence of an E-mail: [email protected] immune ring in the cornea of patients with CMV anterior uveitis. Nodular endothelial lesions are white, medium- Eye (2012) 26, 1152; doi:10.1038/eye.2012.103; sized, nodular lesions surrounded by a translucent halo, published online 25 May 2012 which are significantly associated with CMV infection in cases of chronic anterior uveitis.2,3 Anterior uveitis with ocular hypertension resistant to topical steroid therapy and not clinically suggestive of the herpes group of Sir, virus makes the clinician suspect CMV infection.2 CMV Postoperative eye protection after cataract surgery anterior uveitis responds to ganciclovir, but the relapse rate is high and prolonged therapy may be required. -

Teaching Neuroimages: Central Serous Chorioretinopathy After Corticosteroid Treatment for Optic Neuritis
RESIDENT & FELLOW SECTION Teaching NeuroImages: Central Serous Chorioretinopathy After Corticosteroid Treatment for Optic Neuritis Jennifer Ling, MSc, and Jonathan A. Micieli, MD, CM Correspondence Dr. Micieli Neurology 2021;96:e305-e306. doi:10.1212/WNL.0000000000010807 ® jmicieli@ kensingtonhealth.org Figure Superior Central Serous Chorioretinopathy (CSCR) in the Right Eye and Central CSCR in the Left Eye After Corticosteroid Treatment for Optic Neuritis (A) Color fundus photographs demonstrating a localized superior serous detachment of the retina in the right eye (white arrow) and subfoveal serous detachment of the retina in the left eye (white arrow). (B) Optical coherence tomography of the macula over the localized areas of serous retina detachments demonstrating the subretinal fluid in both eyes (dashed white arrow). A 37-year-old woman presented with a 1-week history of painful vision loss in both eyes from optic MORE ONLINE neuritis. She was treated with intravenous, followed by oral corticosteroids. After she completed Teaching slides intravenous corticosteroids, she developed a new area of blurred vision inferiorly (right eye) and links.lww.com/WNL/ centrally (left eye) secondary to central serous chorioretinopathy (CSCR), which resolved after B213 oral prednisone taper (figure). CSCR is characterized by well-circumscribed serous detachments of the retina and is typically seen after exogenous corticosteroid use. CSCR can be misdiagnosed as optic neuritis1 or develop in patients with optic neuritis after corticosteroid treatment2 and should be kept in the differential diagnosis for worsening vision after corticosteroids. From the Faculty of Medicine (J.L.), University of British Columbia, Vancouver, British Columbia, Canada; Department of Ophthalmology and Vision Sciences (J.A.M.), University of Toronto, Toronto, Ontario, Canada; Division of Neurology (J.A.M.), Department of Medicine, University of Toronto, Toronto, Ontario, Canada; and Kensington Vision and Research Centre (J.A.M.), Toronto, Ontario, Canada. -

Ocular Side Effects of Systemic Drugs.Cdr
ERA’S JOURNAL OF MEDICAL RESEARCH VOL.6 NO.1 Review Article OCULAR SIDE EFFECTS OF SYSTEMIC DRUGS Pragati Garg, Swati Yadav Department of Ophthalmology Era's Lucknow Medical College & Hospital, Sarfarazganj Lucknow, U.P., India-226003 Received on : 06-03-2019 Accepted on : 28-06-2019 ABSTRACT Systemic drugs are frequently administered in persons of all age group Address for correspondence ranging from children to the elderly for various disorders. There has been Dr. Pragati Garg increased reporting of ocular side effects of various systemic drugs in the Department of Ophthalmology past two decades. Some offenders well known are α -2-adrenergic agonists, Era’s Lucknow Medical College & quinine derivatives, β- adrenergic antagonists and antituberculosis drugs. Hospital, Lucknow-226003 Newer systemic drugs causing ocular side effects are being reported in Email: [email protected] available literature. Knowledge regarding these is expected to aid Contact no: +91-9415396506 clinicians in identifying these side effects and the offending drug, thereby, prescribing the appropriate treatment for the condition the patient maybe suffering from without any ocular disturbances. KEYWORDS: Ocular side effects, Systemic drugs. Introduction This article will briefly cover how systemic drugs can Many common systemic medications can affect ocular affect the various ocular structures. tissues and visual function to varying degrees. When a Factors Affecting The Production Of Ocular Side systemic medication is taken to treat another part of the Effects By A Drug body, the eyes frequently are affected. Systemic A) Drug related factors medications can have adverse effects on the eyes that range from dry eye syndrome, keratitis and cataract to (1) The nature of the drug: Absorption of drug in blinding complications of toxic retinopathy and optic body and its pharmacological effects on the body's neuropathy (1). -

Acquired Pendular Nystagmus in Multiple Sclerosis: Clinical Observations and the Role of Optic Neuropathy 263
262 journal ofNeurology, Neurosurgery, and Psychiatry 1993;56:262-267 Acquired pendular nystagmus in multiple J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.56.3.262 on 1 March 1993. Downloaded from sclerosis: clinical observations and the role of optic neuropathy Jason J S Barton, Terry A Cox Abstract identified from the files of patients seen Thirty seven patients with pendular nys- between 1981-90 at the MS Clinic at the tagmus due to multiple sclerosis were University of British Columbia. Only those reviewed. Most developed nystagmus with a "clinically definite" or "clinically prob- later in a progressive phase of the dis- able" diagnosis of MS4 and who had been ease. All had cerebellar signs on exami- examined by a neuro-ophthalmologist were nation and evidence of optic neuropathy. accepted. Two patients were not studied fur- MRI in eight patients showed cerebeliar ther because of insufficient data. or brainstem lesions in seven; the most Data were taken from the first neuro-oph- consistent finding was a lesion in the dor- thalmologic examination noting pendular nys- sal pontine tegmentum. Dissociated nys- tagmus to document the signs most closely tagmus was seen in 18 patients: in these associated with its appearance. Visual acuity the signs of optic neuropathy were often after refraction was assessed with projected asymmetric and the severity correlated Snellen charts. Colour vision was scored with closely with the side with larger oscilla- 16 Ishihara pseudo-isochromatic plates and tions. This suggests that dissociations in optic atrophy was graded on fundoscopy on a acquired pendular nystagmus may be scale of 0 to 4.5 Ocular motility and the due to asymmetries in optic neuropathy amplitude and trajectory of pendular nystag- rather than asymmetries in cerebellar or mus were assessed clinically. -

Pediatric Neuro-Ophthalmology
Pediatric Neuro-Ophthalmology Second Edition Michael C. Brodsky Pediatric Neuro-Ophthalmology Second Edition Michael C. Brodsky, M.D. Professor of Ophthalmology and Neurology Mayo Clinic Rochester, Minnesota USA ISBN 978-0-387-69066-7 e-ISBN 978-0-387-69069-8 DOI 10.1007/978-0-387-69069-8 Springer New York Dordrecht Heidelberg London Library of Congress Control Number: 2010922363 © Springer Science+Business Media, LLC 2010 All rights reserved. This work may not be translated or copied in whole or in part without the written permission of the publisher (Springer Science+Business Media, LLC, 233 Spring Street, New York, NY 10013, USA), except for brief excerpts in connection with reviews or scholarly analysis. Use in connec-tion with any form of information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed is forbidden. The use in this publication of trade names, trademarks, service marks, and similar terms, even if they are not identified as such, is not to be taken as an expression of opinion as to whether or not they are subject to proprietary rights. While the advice and information in this book are believed to be true and accurate at the date of going to press, neither the authors nor the editors nor the publisher can accept any legal responsibility for any errors or omissions that may be made. The publisher makes no warranty, express or implied, with re-spect to the material contained herein. Printed on acid-free paper Springer is part of Springer Science+Business Media (www.springer.com) To the good angels in my life, past and present, who lifted me on their wings and carried me through the storms. -

Amaurosis Fugax (Transient Monocular Or Binocular Vision Loss)
Amaurosis fugax (transient monocular or binocular vision loss) Syndee Givre, MD, PhD Gregory P Van Stavern, MD The next version of UpToDate (15.3) will be released in October 2007. INTRODUCTION AND DEFINITIONS — Amaurosis fugax (from the Greek "amaurosis," meaning dark, and the Latin "fugax," meaning fleeting) refers to a transient loss of vision in one or both eyes. Varied use of common terminology may cause some confusion when reading the literature. Some suggest that "amaurosis fugax" implies a vascular cause for the visual loss, but the term continues to be used when describing visual loss from any origin and involving one or both eyes. The term "transient monocular blindness" is also often used but is not ideal, since most patients do not experience complete loss of vision with the episode. "Transient monocular visual loss" (TMVL) and "transient binocular visual loss" (TBVL) are preferred to describe abrupt and temporary loss of vision in one or both eyes, since they carry no connotation regarding etiology. Transient visual loss, either monocular or binocular, reflects a heterogeneous group of disorders, some relatively benign and others with grave neurologic or ophthalmologic implications. The task of the clinician is to use the history and examination to localize the problem to a region in the visual pathways, identify potential etiologies, and, when indicated, perform a focused battery of laboratory tests to confirm or exclude certain causes. Therapeutic interventions and prognostic implications are specific to the underlying cause. This topic discusses transient visual loss. Other ocular and cerebral ischemic syndromes are discussed separately. APPROACH TO TRANSIENT VISUAL LOSS — By definition, patients with transient visual loss almost always present after the episode has resolved; hence, the neurologic and ophthalmologic examination is usually normal. -
Leber's Hereditary Optic Neuropathy Masquerading As Retinal Vasculitis
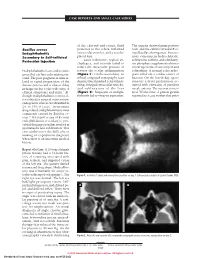
CASE REPORTS AND SMALL CASE SERIES of the choroid and retina, fluid The aspirate showed gram-positive Bacillus cereus posterior to the sclera, inflamed rods, and the culture revealed B ce- Endophthalmitis extraocular muscles, and a nondis- reus/Bacillus thuringiensis. Intrave- Secondary to Self-inflicted placed lens. nous vancomycin hydrochloride, Laser iridotomy, topical cy- ceftriaxone sodium, and clindamy- Periocular Injection cloplegics, and steroids failed to cin phosphate supplemented intra- reduce the intraocular pressure or vitreal injections of vancomycin and Endophthalmitis is an ocular emer- reverse the ocular inflammation ceftazidime. A normal echocardio- gency that can have a devastating out- (Figure 1). On the second day, an gram ruled out a cardiac source of come. The poor prognosis is often re- orbital computed tomography scan bacteria. On the fourth day, spon- lated to rapid progression of the demonstrated marked scleral thick- taneous scleral perforation oc- disease process and a relative delay ening, enlarged extraocular muscles, curred with extrusion of purulent in diagnosis due to the wide array of and subluxation of the lens uveal contents. The eye was eviscer- clinical symptoms and signs.1 Al- (Figure 2). Suspicion of endoph- ated. Weeks later, 2 prison guards though endophthalmitis is most of- thalmitis led to vitreous aspiration. reported to a case worker that prior ten related to surgical intervention, endogenous sources are identified in 2% to 15% of cases.1 Intravenous drug-related endophthalmitis is most commonly caused by Bacillus ce- reus.2,3 We report a case of B cereus endophthalmitis secondary to peri- orbital drug injection that resulted in spontaneous lens subluxation.