Accepted Manuscript
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ladybirds, Ladybird Beetles, Lady Beetles, Ladybugs of Florida, Coleoptera: Coccinellidae1
Archival copy: for current recommendations see http://edis.ifas.ufl.edu or your local extension office. EENY-170 Ladybirds, Ladybird beetles, Lady Beetles, Ladybugs of Florida, Coleoptera: Coccinellidae1 J. H. Frank R. F. Mizell, III2 Introduction Ladybird is a name that has been used in England for more than 600 years for the European beetle Coccinella septempunctata. As knowledge about insects increased, the name became extended to all its relatives, members of the beetle family Coccinellidae. Of course these insects are not birds, but butterflies are not flies, nor are dragonflies, stoneflies, mayflies, and fireflies, which all are true common names in folklore, not invented names. The lady for whom they were named was "the Virgin Mary," and common names in other European languages have the same association (the German name Marienkafer translates Figure 1. Adult Coccinella septempunctata Linnaeus, the to "Marybeetle" or ladybeetle). Prose and poetry sevenspotted lady beetle. Credits: James Castner, University of Florida mention ladybird, perhaps the most familiar in English being the children's rhyme: Now, the word ladybird applies to a whole Ladybird, ladybird, fly away home, family of beetles, Coccinellidae or ladybirds, not just Your house is on fire, your children all gone... Coccinella septempunctata. We can but hope that newspaper writers will desist from generalizing them In the USA, the name ladybird was popularly all as "the ladybird" and thus deluding the public into americanized to ladybug, although these insects are believing that there is only one species. There are beetles (Coleoptera), not bugs (Hemiptera). many species of ladybirds, just as there are of birds, and the word "variety" (frequently use by newspaper 1. -

Studies of the Laboulbeniomycetes: Diversity, Evolution, and Patterns of Speciation
Studies of the Laboulbeniomycetes: Diversity, Evolution, and Patterns of Speciation The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:40049989 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA ! STUDIES OF THE LABOULBENIOMYCETES: DIVERSITY, EVOLUTION, AND PATTERNS OF SPECIATION A dissertation presented by DANNY HAELEWATERS to THE DEPARTMENT OF ORGANISMIC AND EVOLUTIONARY BIOLOGY in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the subject of Biology HARVARD UNIVERSITY Cambridge, Massachusetts April 2018 ! ! © 2018 – Danny Haelewaters All rights reserved. ! ! Dissertation Advisor: Professor Donald H. Pfister Danny Haelewaters STUDIES OF THE LABOULBENIOMYCETES: DIVERSITY, EVOLUTION, AND PATTERNS OF SPECIATION ABSTRACT CHAPTER 1: Laboulbeniales is one of the most morphologically and ecologically distinct orders of Ascomycota. These microscopic fungi are characterized by an ectoparasitic lifestyle on arthropods, determinate growth, lack of asexual state, high species richness and intractability to culture. DNA extraction and PCR amplification have proven difficult for multiple reasons. DNA isolation techniques and commercially available kits are tested enabling efficient and rapid genetic analysis of Laboulbeniales fungi. Success rates for the different techniques on different taxa are presented and discussed in the light of difficulties with micromanipulation, preservation techniques and negative results. CHAPTER 2: The class Laboulbeniomycetes comprises biotrophic parasites associated with arthropods and fungi. -

COLEOPTERA COCCINELLIDAE) INTRODUCTIONS and ESTABLISHMENTS in HAWAII: 1885 to 2015
AN ANNOTATED CHECKLIST OF THE COCCINELLID (COLEOPTERA COCCINELLIDAE) INTRODUCTIONS AND ESTABLISHMENTS IN HAWAII: 1885 to 2015 JOHN R. LEEPER PO Box 13086 Las Cruces, NM USA, 88013 [email protected] [1] Abstract. Blackburn & Sharp (1885: 146 & 147) described the first coccinellids found in Hawaii. The first documented introduction and successful establishment was of Rodolia cardinalis from Australia in 1890 (Swezey, 1923b: 300). This paper documents 167 coccinellid species as having been introduced to the Hawaiian Islands with forty-six (46) species considered established based on unpublished Hawaii State Department of Agriculture records and literature published in Hawaii. The paper also provides nomenclatural and taxonomic changes that have occurred in the Hawaiian records through time. INTRODUCTION The Coccinellidae comprise a large family in the Coleoptera with about 490 genera and 4200 species (Sasaji, 1971). The majority of coccinellid species introduced into Hawaii are predacious on insects and/or mites. Exceptions to this are two mycophagous coccinellids, Calvia decimguttata (Linnaeus) and Psyllobora vigintimaculata (Say). Of these, only P. vigintimaculata (Say) appears to be established, see discussion associated with that species’ listing. The members of the phytophagous subfamily Epilachninae are pests themselves and, to date, are not known to be established in Hawaii. None of the Coccinellidae in Hawaii are thought to be either endemic or indigenous. All have been either accidentally or purposely introduced. Three species, Scymnus discendens (= Diomus debilis LeConte), Scymnus ocellatus (=Scymnobius galapagoensis (Waterhouse)) and Scymnus vividus (= Scymnus (Pullus) loewii Mulsant) were described by Sharp (Blackburn & Sharp, 1885: 146 & 147) from specimens collected in the islands. There are, however, no records of introduction for these species prior to Sharp’s descriptions. -

A Thesis Entitled Influence of Soil-Quality on Coffee-Plant Quality
A Thesis entitled Influence of Soil-Quality on Coffee-Plant Quality and a Complex Tropical Insect Food Web by David J. Gonthier Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Master of Science in Biology (Ecology track) Dr. Stacy Philpott, Committee Chair Dr. Scott Heckathorn, Committee Member Dr. Ivette Perfecto, Committee Member Dr. Patricia Komuniecki, Dean College of Graduate Studies The University of Toledo May 2010 Copyright 2010, David J. Gonthier This document is copyrighted material. Under copyright law, no parts of this document may be reproduced without the expressed permission of the author. An Abstract of Influence of Soil-Quality on Coffee-Plant Quality and a Complex Tropical Insect Food Web by David J. Gonthier Submitted to the Graduate Faculty as partial fulfillment of the requirements for the Master of Science in Biology (Ecology track) The University of Toledo May 2010 Tropical systems are complex, species diverse, and are often regulated by top-down forces (higher trophic levels control lower trophic levels). In many ecosystems insects, especially herbivores and their mutualists, may be strongly affected by plant quality and other bottom-up controls (nutrient availability, plant genetic variation, ect.). Yet few have asked how plant quality (nutritional and defensive plant traits) can contribute to the population regulation and the complexity of these systems. In this thesis, I investigate the importance of soil-quality to both the elemental and secondary metabolite content in coffee and ask how changes to plant quality can influence hemipteran herbivores, their ant-mutualists, predators, and insect communities in a tropical coffee agroecosystem. -

PROCEEDINGS of the 12Th ANNUAL 2012 ZEBRA CHIP REPORTING SESSION
PROCEEDINGS OF THE 12th ANNUAL 2012 ZEBRA CHIP REPORTING SESSION F. Workneh, A. Rashed, and C. M. Rush Editors San Antonio, TX Oct. 30 – Nov. 2, 2012 PREFACE Zebra chip of potato (ZC) was first documented from potato fields around Saltillo, Mexico in 1994, and in 2000 it was identified in South Texas. In the USA, the disease initially was considered a regional problem in South Texas, but by 2006 ZC had been identified from all potato production areas in Texas, and also in Arizona, California, Colorado, Kansas, Nebraska, Nevada, and New Mexico. Outside of the USA, ZC has been reported from Guatemala, Honduras, Mexico and New Zealand. Early studies of ZC were hampered by lack of knowledge concerning disease etiology, but in 2007, the potato psyllid, Bactericera cockerelli, was definitively associated with ZC and in 2008 two independent studies reported the association of Candidatus Liberibacter spp. with ZC. It now has been repeatedly demonstrated that transmission of Candidatus Liberibacter solanacearum by the potato psyllid results in diagnostic symptoms of ZC, while infestations by potato psyllids without Candidatus Liberibacter solanacearum do not cause ZC. However, questions still exist concerning the effect of pathogen and vector variability on disease severity. Soon after ZC was first identified in South Texas, representatives from Frito Lay, approximately four farmers and two plant pathologists met to discuss how to deal with the new disease. Grower sponsored research projects were initiated the next year, and the same small group met again, after the 2001 harvest, and in an informal setting presented their findings and observations. This meeting constituted the first ZC reporting session. -

Insects and Related Arthropods Associated with of Agriculture
USDA United States Department Insects and Related Arthropods Associated with of Agriculture Forest Service Greenleaf Manzanita in Montane Chaparral Pacific Southwest Communities of Northeastern California Research Station General Technical Report Michael A. Valenti George T. Ferrell Alan A. Berryman PSW-GTR- 167 Publisher: Pacific Southwest Research Station Albany, California Forest Service Mailing address: U.S. Department of Agriculture PO Box 245, Berkeley CA 9470 1 -0245 Abstract Valenti, Michael A.; Ferrell, George T.; Berryman, Alan A. 1997. Insects and related arthropods associated with greenleaf manzanita in montane chaparral communities of northeastern California. Gen. Tech. Rep. PSW-GTR-167. Albany, CA: Pacific Southwest Research Station, Forest Service, U.S. Dept. Agriculture; 26 p. September 1997 Specimens representing 19 orders and 169 arthropod families (mostly insects) were collected from greenleaf manzanita brushfields in northeastern California and identified to species whenever possible. More than500 taxa below the family level wereinventoried, and each listing includes relative frequency of encounter, life stages collected, and dominant role in the greenleaf manzanita community. Specific host relationships are included for some predators and parasitoids. Herbivores, predators, and parasitoids comprised the majority (80 percent) of identified insects and related taxa. Retrieval Terms: Arctostaphylos patula, arthropods, California, insects, manzanita The Authors Michael A. Valenti is Forest Health Specialist, Delaware Department of Agriculture, 2320 S. DuPont Hwy, Dover, DE 19901-5515. George T. Ferrell is a retired Research Entomologist, Pacific Southwest Research Station, 2400 Washington Ave., Redding, CA 96001. Alan A. Berryman is Professor of Entomology, Washington State University, Pullman, WA 99164-6382. All photographs were taken by Michael A. Valenti, except for Figure 2, which was taken by Amy H. -

Wax Structures of Scymnus Louisianae Attenuate Aggression from Aphid-Tending Ants
CHEMICAL ECOLOGY Wax Structures of Scymnus louisianae Attenuate Aggression From Aphid-Tending Ants EZRA G. SCHWARTZBERG,1,2 KENNETH F. HAYNES,1 DOUGLAS W. JOHNSON,1 1 AND GRAYSON C. BROWN Environ. Entomol. 39(4): 1309Ð1314 (2010); DOI: 10.1603/EN09372 ABSTRACT The cuticular wax structures of Scymnus louisianae J. Chapin larvae were investigated as a defense against ant aggression by Lasius neoniger Emery. The presence of wax structures provided signiÞcant defense against ant aggression compared with denuded larvae in that these structures attenuated the aggressive behavior of foraging ants. Furthermore, reapplication of wax dissolved in hexane partially restored defenses associated with intact structures, showing an attenuation of aggression based in part on cuticular wax components rather than solely on physical obstruction to ant mouthparts. KEY WORDS Scymnus louisianae, Aphis glycines, Lasius neoniger, ant-tending Many ants tend aphids and use the collected honeydew wax in promoting physical obstruction to the mouthparts as a sugar source. In return, the aphids beneÞt from the of aphid-tending ants, little has been done to elucidate tending ants (Way 1963, Ho¨lldobler and Wilson 1990). In the role that wax plays in eliciting avoidance behavior or these systems, aphids can beneÞt through reduced pre- in attenuating aggression. SpeciÞcally, physical or chem- dation (Banks 1962) and increased Þtness (Flatt and ical mimicry, camoußage, or repellency has not been Weisser 2000). The relationship between ants and aphids looked at as a potential means of avoidance of ant ag- is often a mutualistic one but ranges from obligatory to gression in the Scymnus beetles. It has been suggested facultative (Dixon 1998). -

Taxonomic Studies of Family Coccinellidae (Coleoptera) of Gilgit-Baltistan, Pakistan by Muhammad Ashfaque Doctor of Philosophy I
TAXONOMIC STUDIES OF FAMILY COCCINELLIDAE (COLEOPTERA) OF GILGIT-BALTISTAN, PAKISTAN BY MUHAMMAD ASHFAQUE A dissertation submitted to the University of Agriculture, Peshawar in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY IN AGRICULTURE (PLANT PROTECTION) DEPARTMENT OF PLANT PROTECTION FACULTY OF CROP PROTECTION SCIENCES The UNIVERSITY OF AGRICULTURE, PESHAWAR KHYBER PAKHTUNKHWA-PAKISTAN DECEMBER, 2012 TAXONOMIC STUDIES OF FAMILY COCCINELLIDAE (COLEOPTERA) OF GILGIT-BALTISTAN, PAKISTAN BY MUHAMMAD ASHFAQUE A dissertation submitted to the University of Agriculture, Peshawar in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY IN AGRICULTURE (PLANT PROTECTION) Approved by: _________________________ Chairman, Supervisory Committee Prof. Dr. Farman Ullah Department of Plant Protection _________________________ Co-Supervisor Dr. Muhammad Ather Rafi PSO/PL, NIM, IPEP, NARC Islamabad _________________________ Member Prof. Dr. Ahmad ur Rahman Saljoqi Department of Plant Protection _________________________ Member Prof. Dr. Sajjad Ahmad Department of Entomology _________________________ Chairman and Convener Board of Studies Prof. Dr. Ahmad ur Rahman Saljoqi _________________________ Dean, Faculty of Crop Protection Sciences Prof. Dr. Mian Inayatullah _________________________ Director, Advanced Studies and Research Prof. Dr. Farhatullah DEPARTMENT OF PLANT PROTECTION FACULTY OF CROP PROTECTION SCIENCES The UNIVERSITY OF AGRICULTURE, PESHAWAR KHYBER PAKHTUNKHWA-PAKISTAN DECEMBER, 2012 -

Status Report on Arthropod Predators of the Asian Citrus Psyllid in Mexico
Status report on arthropod predators of the Asian Citrus Psyllid in Mexico Esteban Rodríguez-Leyva Colegio de Postgraduados, Montecillo, Texcoco, MEXICO. Importance of Citrus in Mexico 525 000 ha 23 States Citrus production 6.7 million ton/year 68% Orange, 20% Mexican lime, 5% Persian lime 2.5% Grapefruit , 2.5% Tangerine The insect problem Diaphorina citri in Mexico (www.senasica.gob.mx) Searching for natural enemies of ACP on citrus trees and orange jessamine Coccinellidae Azya orbigera Brachiacantha decora Chilocorus cacti Cycloneda sanguinea Harmonia axyridis Hippodamia convergens Olla v-nigrum López- Arroyo et al. 2008 Marín 2009, personal com. Sánchez-González & Arredondo-Bernal 2009, personal com. Gaona-García et al. 2009 Chrysopidae Chrysoperla rufilabris C. comanche C. externa Ceraeochrysa sp. nr. cincta C. valida López- Arroyo et al. 2008 Cortez-Mondaca et al. 2008 Syrphidae Allographa obliqua Pseudodoros clavatus Toxomerus marginatus Toxomerus politus Miscelaneous predators of ACP Spiders, Wasps (Vespidae), Ants (Ponerinae) López- Arroyo et al. 2008 Sánchez-González & Arredondo-Bernal 2009, personal com. Abundance of ladybirds (Coccinellidae) predators on citrus trees, at Nuevo Leon (northeast), Mexico. (Insect net, two sides of each mature tree, n=30). López-Arroyo, 2010, personal com. Abundance of lacewings (Chrysopidae) predators on citrus trees, at Nuevo Leon (northeast), Mexico. (Insect net two sides of each mature tree, n=30). López-Arroyo, 2010, personal com. Abundance of lacewings (Chrysopidae) predators on citrus trees, at Nuevo Leon (northeast), Mexico. (Insect net two sides of each mature tree, n=30). López-Arroyo, 2010, personal com. Abundance of predators (specially C. rufilabris) on Diaphorina citri on citrus trees at Nuevo Leon (northeast), Mexico. -

Integrative Taxonomy Reveals Hidden Species Within a Common Fungal Parasite of Ladybirds Received: 19 April 2018 Danny Haelewaters 1,2, André De Kesel3 & Donald H
www.nature.com/scientificreports OPEN Integrative taxonomy reveals hidden species within a common fungal parasite of ladybirds Received: 19 April 2018 Danny Haelewaters 1,2, André De Kesel3 & Donald H. Pfster1 Accepted: 8 October 2018 Our understanding of fungal diversity is far from complete. Species descriptions generally focus on Published: xx xx xxxx morphological features, but this approach may underestimate true diversity. Using the morphological species concept, Hesperomyces virescens (Ascomycota, Laboulbeniales) is a single species with global distribution and wide host range. Since its description 120 years ago, this fungal parasite has been reported from 30 species of ladybird hosts on all continents except Antarctica. These host usage patterns suggest that H. virescens could be made up of many diferent species, each adapted to individual host species. Using sequence data from three gene regions, we found evidence for distinct clades within Hesperomyces virescens, each clade corresponding to isolates from a single host species. We propose that these lineages represent separate species, driven by adaptation to diferent ladybird hosts. Our combined morphometric, molecular phylogenetic and ecological data provide support for a unifed species concept and an integrative taxonomy approach. What is a species? Tis is a perennial question in evolutionary biology. Te answer is complex and has been intensely argued for decades. Diferent species concepts corresponding to multiple biological properties pro- vide a means to recognize, delineate and describe species. Tese properties include diferences in morphological traits, nucleotide divergence and monophyly, reproductive isolation, ecological niches or adaptive zones, mate recognition or mating systems, geographic range, exclusive coalescence of alleles, etc. However, biologists from various research felds have advocated diferent and sometimes incompatible species concepts, leading to varying conclusions regarding delimitation of species and their numbers. -

La Cuenca Del Lago De Cuitzeo, Michoacán
CONTRIBUCIONES PARA EL DESARROLLO SOSTENIBLE DE LA CUENCA DEL LAGO DE CUITZEO, MICHOACÁN Editores Técnicos Miguel Bravo Espinosa Gerardo Barrera Camacho Manuel E. Mendoza J. Trinidad Sáenz Reyes Fernando Bahena Juárez Rubén Sánchez Martínez CONTRIBUCIONES PARA EL DESARROLLO SOSTENIBLE DE LA CUENCA DEL LAGO DE CUITZEO, MICHOACÁN Editores Técnicos Miguel Bravo Espinosa Gerardo Barrera Camacho Manuel E. Mendoza J. Trinidad Sáenz Reyes Fernando Bahena Juárez Rubén Sánchez Martínez INSTITUTO NACIONAL DE UNIVERSIDAD NACIONAL AUTÓNOMA DE INVESTIGACIONES FORESTALES, MÉXICO AGRÍCOLAS Y PECUARIAS CENTRO DE INVESTIGACIONES EN CENTRO DE INVESTIGACIÓN REGIONAL GEOGRAFÍA AMBIENTAL PACIFICO CENTRO CAMPO EXPERIMENTAL URUAPAN Marzo de 2012 CONTRIBUCIONES PARA EL DESARROLLO SOSTENIBLE DE LA CUENCA DEL LAGO DE CUITZEO, MICHOACÁN. Bravo-Espinosa, M., G. Barrera-Camacho, M.E. Mendoza, J.T. Sáenz, F. Bahena-Juárez y R. Sánchez-Martínez (eds.). 2012. Contribuciones para el desarrollo sostenible de la cuenca del Lago de Cuitzeo, Michoacán. INIFAP-Campo Experimental Uruapan. Uruapan, Michoacán. UNAM- Centro de Investigaciones en Geografía Ambiental. Morelia, Michoacán, México. Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias. Av. Progreso Núm. 5 Col. Barrio de Santa Catarina. Delegación Coyoacán. C.P. 04010 México, D.F. Tel. (01 55) 38 71 87 00 www.inifap.gob.mx Correo-e: [email protected] Centro de Investigación Regional del Pacífico Centro. Campo Experimental Uruapan. Av. Latinoamericana Núm. 1101. Col. Revolución. Uruapan, Michoacán, México. Universidad Nacional Autónoma de México (Campus Morelia) Centro de Investigaciones en Geografía Ambiental Antigua Carretera a Pátzcuaro 8701 Col. ExHacienda de San José de la Huerta C.P. 58190, Morelia, Michoacán, México ISBN: 978-607-02-2914-5 Primera edición 2012. -
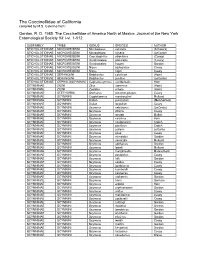
The Coccinellidae of California Compiled by M.S
The Coccinellidae of California compiled by M.S. Caterino from: Gordon, R. D. 1985. The Coccinellidae of America North of Mexico. Journal of the New York Entomological Society 93: i-vi, 1-912. SUBFAMILY TRIBE GENUS SPECIES AUTHOR STICHOLOTIDINAE MICROWEISEINI Microweisea suturalis (Schwarz) STICHOLOTIDINAE MICROWEISEINI Microweisea misella (LeConte) STICHOLOTIDINAE MICROWEISEINI Coccidophilus atronitens (Casey) STICHOLOTIDINAE MICROWEISEINI Gnathoweiea planiceps (Casey) STICHOLOTIDINAE MICROWEISEINI Gnathoweiea hageni Gordon STICHOLOTIDINAE MICROWEISEINI Nipus biplagiatus Casey STICHOLOTIDINAE MICROWEISEINI Nipus niger Casey STICHOLOTIDINAE SERANGIINI Delphastus catalinae (Horn) STICHOLOTIDINAE SERANGIINI Delphastus pusillus (LeConte) STICHOLOTIDINAE CEPHALOSCYMNINI Cephaloscymnus occidentalis Horn SCYMNINAE ZILINI Zilus aterrimus (Horn) SCYMNINAE ZILINI Zagloba ornata (Horn) SCYMNINAE STETHORINI Stethorus punctum picipes Casey SCYMNINAE SCYMNINI Cryptolaemus montrouzieri Mulsant SCYMNINAE SCYMNINI Didion punctatum (Melsheimer) SCYMNINAE SCYMNINI Didion longulum Casey SCYMNINAE SCYMNINI Scymnus nebulosus (LeConte) SCYMNINAE SCYMNINI Scymnus dificilis Casey SCYMNINAE SCYMNINI Scymnus fenderi Malkin SCYMNINAE SCYMNINI Scymnus caurinus Horn SCYMNINAE SCYMNINI Scymnus coniferarum Crotch SCYMNINAE SCYMNINI Scymnus pacificus Crotch SCYMNINAE SCYMNINI Scymnus pallens LeConte SCYMNINAE SCYMNINI Scymnus gilae Casey SCYMNINAE SCYMNINI Scymnus mimoides Gordon SCYMNINAE SCYMNINI Scymnus cervicalis Mulsant SCYMNINAE SCYMNINI Scymnus apithanus Gordon