A Field Based Evaluation of Adverse Events Following Menafrivac®Vaccine Delivered in a Controlled Temperature Chain (CTC) Approach in Benin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Trade Setting and Market Structure (1970S - 1990S)
UvA-DARE (Digital Academic Repository) Trade and Traders. The Making of the Cattle Market in Benin Quarles van Ufford, P.E.J. Publication date 1999 Link to publication Citation for published version (APA): Quarles van Ufford, P. E. J. (1999). Trade and Traders. The Making of the Cattle Market in Benin. Thela Thesis. General rights It is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), other than for strictly personal, individual use, unless the work is under an open content license (like Creative Commons). Disclaimer/Complaints regulations If you believe that digital publication of certain material infringes any of your rights or (privacy) interests, please let the Library know, stating your reasons. In case of a legitimate complaint, the Library will make the material inaccessible and/or remove it from the website. Please Ask the Library: https://uba.uva.nl/en/contact, or a letter to: Library of the University of Amsterdam, Secretariat, Singel 425, 1012 WP Amsterdam, The Netherlands. You will be contacted as soon as possible. UvA-DARE is a service provided by the library of the University of Amsterdam (https://dare.uva.nl) Download date:07 Oct 2021 Trade setting and market structure (1970s - 1990s) The present chapter pursues the historical line embarked upon in Chapter 3. It deals with the period between the 1970s and the 1990s. Its specific focus is on the developments and changes in the trade setting and in the market structure which occurred over this period. -

Volume I : Etat Des Lieux
République Burkina Faso République du Bénin du Niger Fonds européen de développement Programme Régional Parc W / ECOPAS (Ecosystèmes Protégés en Afrique Sahélienne) 7 ACP RPR 742 "Plan d'Aménagement et de Gestion de la Réserve de Biosphère Transfrontalière W - 2006-2010" Volume I : Etat des lieux VERSION FINALE Mai 2005 Programme Régional Parc W / ECOPAS 7 ACP RPR 742 "Plan d'Aménagement et de Gestion de la Réserve de Biosphère Transfrontalière W - 2006-2010" janvier - novembre 2004 Réalisé par le Programme Régional Parc W / ECOPAS en collaboration avec les Administrations de tutelles et les Communautés riveraines , avec l’appui technique de : Alain BILLAND, Marie Noël DE VISSCHER, KIDJO Ferdinand Claude, Albert COMPAORE, Amadou BOUREIMA, Alexandra MOREL, Laye CAMARA ; Frank CZESNIK, Nestor René AHOYO ADJOVI Adresse : Consultant Bureau de Coordination du Programme Consortium ECOPAS : Régional Parc W / ECOPAS Agrer S.A., Agriconsulting S.p.A., 01 BP 1607 CIRAD, GFA Terra Systems GmbH, Imm. PPI BF face BIB avenue du Temple Deutsche Gesellschaft für Technische Ouagadougou 01 Zusammenarbeit (GTZ) GmbH, Burkina Faso Tél./Fax: (+226) 335261 s/c GFA Terra Systems GmbH [email protected] Eulenkrugstr. 82 D – 22359 Hamburg Tel.: (+49)40-60306-111 Fax: (+49)40-60306-119 E-Mail: [email protected] Plan d'Aménagement et de Gestion de la RBT W (Bénin, Burkina Faso, Niger) Etat des lieux – Version finale ETAT DES LIEUX DE LA RESERVE DE BIOSPHERE TRANSFRONTALIERE W Sommaire 1 LISTE DES ABREVIATIONS .............................................................................................. 10 2 ELABORATION DU PLAN D’AMENAGEMENT ET DE GESTION............................... 14 3 HISTORIQUE........................................................................................................................ 16 3.1 HISTORIQUE DE LA PROTECTION DE LA NATURE ET DES PARCS NATIONAUX...................... -

The Cross-Border Transhumance in West Africa Proposal for Action Plan
Food and Agricultural Organization of the United Nations in collaboration with Economic Community of West African States The cross-border transhumance in West Africa Proposal for Action Plan June 2012 TABLE OF CONTENT TABLE OF CONTENT ...................................................................................................................................... 2 EXECUTIVE SUMMARY.................................................................................................................................. 5 Acronyms and Abbreviations ....................................................................................................................... 7 1. Introduction ........................................................................................................................................ 10 2. Background of livestock in West Africa .................................................................................................. 12 2.1. Increasing livestock numbers .......................................................................................................... 12 2.2. Many animal breeds but some endangered ................................................................................... 12 2.3. Livestock production systems in West Africa .................................................................................. 15 2.3.1. Pastoral systems ....................................................................................................................... 15 2.3.2. Urban and peri-urban livestock -

PROSAF Transition Phase Final Report January 11, 2004 to October 31, 2005
PROSAF Transition Phase Final Report January 11, 2004 to October 31, 2005 for USAID/Benin Contract No. 680-C-00-04-00039-00 Submitted by University Research Co., LLC November 30, 2005 Distribution Rudolph Thomas, USAID/ Benin Francine Nicoué, USAID/ Benin Martin Fischer, USAID Modupe Broderick, USAID/ Benin Pascal Zinzindohoue, USAID/ Benin ABPF/Cotonou Souley Abdoulaye DDSP/Borgou/Alibori Médecins Coordinateurs, Borgou/Alibori Siri Wood, PATH Jim Cawley, CLUSA Jim Alrutz, CLUSA Table of Contents ACROYNM LIST 1. INTRODUCTION....................................................................................................................................1 2. INSTITUTIONALIZATION APPROACH...........................................................................................1 3. RESULTS .................................................................................................................................................3 3.1 Data Summarizing Main Acheivements…………………………………………………….3 4. MAIN ACHEIVEMENTS BY INTERMEDIATE RESULT ...............................................................3 4.1 Intermediate Result 1: Improved Policy Environment…………………………....………...3 4.2 Intermediate Result 2: Increased Access to Family Health Products and Services……… 11 4.3 Intermediate Result 3: Improved Quality of Services……………………………………..17 4.4 Intermediate Result 4: Increased Demand for Family Health Services and Prevention Measures…………………………………………………………………………..……….31 5. CONCLUSION.........................................................................................................................................38 -

Par L'équipe Conjointe BASICS Et DDSPSCFIB 4 Septembre 1997
RAPPORT PRÉLIMINAIRE PRÉSENTÉ À LA DIRECTION DÉPARTEMENTALE DE LA SANTÉ, DE LA PROTECTION SOCIALE ET DE LA CONDITION FÉMININE (DDSPSCF) DE BORGOU SUR LES INTERVENTIONS DU "PAQUET MINIMUM DES ACTIVITÉS"! NUTRITION (pMAIN) Par l'équipe conjointe BASICS et DDSPSCFIB 4 septembre 1997 Tina Sanghvi Serigne Diène accompagné par Léopold Fakambi BASICS Technical Directive: 000 BN 01 017 USAID Contract Number: HRN-C-00-93-00031-00 TABLE OF CONTENTS ABRÉVIATIONS 1. INTRODUCTION ....................................................... 1 II BUT ET OBJECTIFS .................................................... 1 III ORGANISATION ET MÉTHODOLOGIE DE LA VISITE DE TERRAIN .......... 2 IV RÉSULTATS DE LA VISITE DE TERRAIN ................................. 2 V. PROCHAINES ÉTAPES DE LA MISE EN OEUVRE DU PMAINUTRITION DANS LE DÉPARTEMENT DE BORGOU : PROPOSITIONS POUR LES 12 PROCHAINS MOIS ................................................................. 8 A. ACTIONS COMMUNES À TOUTES LES COMPOSANTES ........ 8 B. PROMOTION DE L'ALLAITEMENT MATERNEL ............... 8 C. L ' ALIMENTATION COMPLÉMENTAIRE (BONNES PRATIQUES DE SEVRAGES) ................................ 8 D. VITAMINE A .............................................. 9 E. LA SUPPLÉMENTATION EN FER DE LA FEMME ENCEINTE .... 9 F. LA PROMOTION DE LA CONSOMMATION DU SEL IODÉ ....... 9 G. PRISE EN CHARGE NUTRITIONNELLE DES MALADIES DEL'ENFANT ............................................. 9 LISTE DES TABLEAUX ET FIGURES Table 1. Overview of PMAIN Protocols in Health Services Table 2. Situation Nutritionnelle et Sanitaire des Enfants dans le Département du Borgou, EDS 1996 Table 3. Health Personnel in Borgou Department Table 4. List of Health Facilities Visited Table 5. Current Situation and Gaps in PMAIN Components in Borgou Table 6. Number Enrolled in the CPS Program in Borgou Figure 1. Contribution of Malnutrition to Child Deaths in Benin Figure 2. Prevalence of Malnutrition by Department Figure 3. Malnutrition by Age in Benin Figure 4. -

Adaptation Strategies of Cattle Farmers in the Dry and Sub-Humid Tropical Zones of Benin in the Context of Climate Change
Heliyon 6 (2020) e04373 Contents lists available at ScienceDirect Heliyon journal homepage: www.cell.com/heliyon Research article Adaptation strategies of cattle farmers in the dry and sub-humid tropical zones of Benin in the context of climate change Yaya Idrissou a,*, Alassan Seidou Assani a, Mohamed Nasser Baco b, Afouda Jacob Yabi c, Ibrahim Alkoiret Traore a a Laboratoire d’Ecologie, Sante et Production Animales (LESPA), Faculted’Agronomie (FA), Universite de Parakou, 01 BP 123, Parakou, Benin b Laboratoire Societe-Environnement (LaSEn), Faculte d'Agronomie, Universite de Parakou, 01 BP 123, Parakou, Benin c Laboratoire d’Analyse et de Recherches sur les Dynamiques Economiques et Sociales (LARDES), Faculted’Agronomie, Universite de Parakou, BP 123, Parakou, Benin ARTICLE INFO ABSTRACT Keywords: Cattle farming is directly impacted by climate change (CC), as it utilizes resources whose seasonality and pro- Adaptation strategies ductivity are strongly climate-dependent. Farmers respond to the negative influence of CC by implementing Climate change different adaptation strategies, where choices are informed by many factors. This study aims at analyzing the Livestock adaptation strategies of cattle farmers in the dry tropical zone (DTZ) and sub-humid tropical zone (STZ) of Benin Benin with regard to climate change, as well as the determinants for the choice of these strategies. For that matter, 360 Agricultural science Biological sciences cattle farmers were surveyed. Data collected were related to the demographic and socio-economic characteristics Earth sciences of the cattle farmers, their perception and adaptation to CC. The data collected were subjected to frequency Environmental science analysis and binary logistic regression. The results showed that livestock farmers were partly aware of climate Social sciences related with CC, especially the increase of temperature. -

Déterminants Des Itinéraires De Transhumance À La Périphérie De La Réserve De Biosphère Transfrontalière Du W Au Bénin
Kperou Gado et al., J. Appl. Biosci. 2020 Déterminants des itinéraires de transhumance à la périphérie de la réserve de biosphère transfrontalière du W au Bénin Journal of Applied Biosciences 152: 15650 - 15666 ISSN 1997-5902 Déterminants des itinéraires de transhumance à la périphérie de la réserve de biosphère transfrontalière du W au Bénin Byll O. KPEROU GADO* 1&4 , Ismaïla TOKO IMOROU 2, Ousséni AROUNA 3, Habirou SIDI IMOROU 1 et Madjidou OUMOROU 4 1 Ministère de l’Agriculture, de l’Elevage et de la Pêche, 03 BP : 2900 Cotonou, Bénin. 2 Laboratoire de Cartographie "LaCarto"/Université d’Abomey-Calavi, 10 BP 1082 Cotonou-Houéyiho, Bénin. 3 Ecole Nationale Supérieure des Travaux Publics (ENSTP), Université Nationale des Sciences, Technologies, Ingénierie et Mathématiques d'Abomey (UNSTIM), BP : 2282, Goho Abomey, Bénin. 4 Laboratoire de Recherche en Biologie Appliquée (LARBA), Unité de Recherche en Phytosociologie, Espaces Protégés et Pastoraux, Agro-écosystèmes, Conservation et valorisation des espèces endogènes (PEPPAC), Ecole Polytechnique d’Abomey-Calavi, BP 2009 Cotonou, Bénin. * Auteur correspondant - Email : [email protected] Original submitted in on 1 th June 2020. Published online at www.m.elewa.org/journals/ on 31 st August 2020 https://doi.org/10.35759/JABs.152.5 RESUME Objectifs : la présente étude vise à identifier les facteurs concourant à la définition des itinéraires de transhumance et analyser la perception des éleveurs transhumants sur les déterminants de ces itinéraires en vue d’une amélioration de la prise de décision dans la gestion des écosystèmes pâturés. Méthodologie et résultats : la démarche méthodologique a consisté à cartographier, avec le logiciel ArcGIS 10.4, les infrastructures pastorales et à faire une enquête socioéconomique de la perception des transhumants sur la situation et la gestion des couloirs. -

Plan Directeur D'electrification Hors Réseau
Plan Directeur d’Electrification Hors Réseau Prévision de la demande - 2018 Annexe 3 Etude pour la mise en place d’un environnement propice à l’électrification hors-réseau Présenté par : Projet : Accès à l’Électricité Hors Réseau Activité : Etude pour la mise en place d’un environnement propice à l’électrification hors-réseau Contrat n° : PP1-CIF-OGEAP-01 Client : Millennium Challenge Account-Bénin II (MCA-Bénin II) Financement : Millennium Challenge Corporation (MCC) Gabriel DEGBEGNI - Coordonnateur National (CN) Dossier suivi par : Joel AKOWANOU - Directeur des Opérations (DO) Marcel FLAN - Chef du Projet Energie Décentralisée (CPED) Groupement : IED - Innovation Energie Développement (Fr) PAC - Practical Action Consulting Ltd (U.K) 2 chemin de la Chauderaie, 69340 Francheville, France Consultant : Tel: +33 (0)4 72 59 13 20 / Fax: +33 (0)4 72 59 13 39 E-mail : [email protected] / [email protected] Site web: www.ied-sa.fr Référence IED : 2016/019/Off Grid Bénin MCC Date de démarrage : 21 nov. 2016 Durée : 18 mois Rédaction du document : Version Note de cadrage Version 1 Version 2 FINAL Date 26 juin 2017 14 juillet 2017 07 septembre 2017 17 juin 2017 Rédaction Jean-Paul LAUDE, Romain FRANDJI, Ranie RAMBAUD Jean-Paul LAUDE - Chef de projet résident Relecture et validation Denis RAMBAUD-MEASSON - Directeur de projet Off-grid 2017 Bénin IED Prévision de la Demande Localités non électrifiées Scenario 24h Première année Moyen-terme Horizon Population Conso. Pointe Part do- Conso. (kWh) Pointe Part do- Conso. Pointe Part do- Code Localité -

Programme D'actions Du Gouvernement 2016-2021
PROGRAMME D’ACTIONS DU GOUVERNEMENT 2016-2021 ÉTAT DE MISE EN œuvre AU 31 MARS 2019 INNOVATION ET SAVOIR : DÉVELOPPER UNE ÉCONOMIE DE L’INNOVATION ET DU SAVOIR, SOURCE D’EMPLOIS ET DE CROISSANCE – © BAI-AVRIL 2019 A PROGRAMME D’ACTIONS DU GOUVERNEMENT 2016-2021 ÉTAT DE MISE EN œuvre AU 31 MARS 2019 2 Sommaire 1. Avant-propos p. 4 2. Le PAG en bref p. 8 3. État d’avancement des réformes p. 14 4. Mise en œuvre des projets p. 26 TOURISME p. 30 AGRICULTURE p. 44 INFRASTRUCTURES p. 58 NUMÉRIQUE p. 74 ÉLECTRICITÉ p. 92 CADRE DE VIE p. 110 EAU POtaBLE p. 134 PROTECTION SOCIALE p. 166 CITÉ INTERNatIONALE DE L’INNOVatION ET DU SaVoir – SÈMÈ CITY p. 170 ÉDUCatION p. 178 SPORT ET CULTURE p. 188 SaNTÉ p. 194 5. Mobilisation des ressources p. 204 6. Annexes p. 206 Annexe 1 : ÉLECTRICITÉ p. 210 Annexe 2 : CADRE DE VIE p. 226 Annexe 3 : EAU POTABLE p. 230 SOMMAIRE – © BAI-AVRIL 2019 3 1 4 RÉCAPITULATIF DES RÉFORMES MENÉES – © BAI-AVRIL 2019 Avant-propos RÉCAPITULATIF DES RÉFORMES MENÉES – © BAI-AVRIL 2019 5 Avant-propos Les équipes du Président Patrice TALON poursuivent du PAG. Il convient de souligner que ces fonds ont été résolument la mise en œuvre des projets inscrits dans affectés essentiellement au financement des infrastruc- le Programme d’Actions du Gouvernement PAG 2016– tures nécessaires pour impulser l’investissement privé 2021. Dans le présent document, l’état d’avancement (énergie, routes, internet haut débit, attractions, amé- de chacun des projets phares est fourni dans des fiches nagement des plages,…). -

Laws of Attraction Northern Benin and Risk of Violent Extremist Spillover
Laws of Attraction Northern Benin and risk of violent extremist spillover CRU Report Kars de Bruijne Laws of Attraction Northern Benin and risk of violent extremist spillover Kars de Bruijne CRU Report June 2021 This is a joint report produced by the Conflict Research Unit of Clingendael – the Netherlands Institute of International Relations in partnership with the Armed Conflict Location & Event Data Project (ACLED). June 2021 © Netherlands Institute of International Relations ‘Clingendael’. Cover photo: © Julien Gerard Unauthorized use of any materials violates copyright, trademark and / or other laws. Should a user download material from the website or any other source related to the Netherlands Institute of International Relations ‘Clingendael’, or the Clingendael Institute, for personal or non-commercial use, the user must retain all copyright, trademark or other similar notices contained in the original material or on any copies of this material. Material on the website of the Clingendael Institute may be reproduced or publicly displayed, distributed or used for any public and non-commercial purposes, but only by mentioning the Clingendael Institute as its source. Permission is required to use the logo of the Clingendael Institute. This can be obtained by contacting the Communication desk of the Clingendael Institute ([email protected]). The following web link activities are prohibited by the Clingendael Institute and may present trademark and copyright infringement issues: links that involve unauthorized use of our logo, framing, inline links, or metatags, as well as hyperlinks or a form of link disguising the URL. About the author Kars de Bruijne is a Senior Research Fellow with the Clingendael’s Conflict Research Unit and a former Senior Researcher at ACLED. -
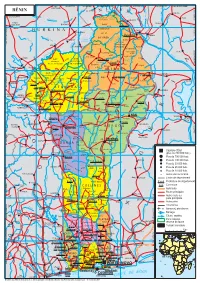
BENIN-2 Cle0aea97-1.Pdf
1° vers vers BOTOU 2° vers NIAMEY vers BIRNIN-GAOURÉ vers DOSSO v. DIOUNDIOU vers SOKOTO vers BIRNIN KEBBI KANTCHARI D 4° G vers SOKOTO vers GUSAU vers KONTAGORA I E a BÉNIN N l LA TAPOA N R l Pékinga I o G l KALGO ER M Rapides a vers BOGANDÉ o Gorges de de u JE r GA Ta Barou i poa la Mékrou KOULOU Kompa FADA- BUNZA NGOURMA DIAPAGA PARC 276 Karimama 12° 12° NATIONAL S o B U R K I N A GAYA k o TANSARGA t U DU W o O R Malanville KAMBA K Ka I bin S D É DU NIGER o ul o M k R G in u a O Garou g bo LOGOBOU Chutes p Guéné o do K IB u u de Koudou L 161 go A ZONE vers OUAGADOUGOU a ti r Kandéro CYNÉGÉTIQUE ARLI u o KOMBONGOU DE DJONA Kassa K Goungoun S o t Donou Béni a KOKO RI Founougo 309 JA a N D 324 r IG N a E E Kérémou Angaradébou W R P u Sein PAMA o PARC 423 ZONE r Cascades k Banikoara NATIONAL CYNÉGÉTIQUE é de Sosso A A M Rapides Kandi DE LA PENDJARI DE L'ATAKORA Saa R Goumon Lougou O Donwari u O 304 KOMPIENGA a Porga l é M K i r A L I B O R I 11° a a ti A j 11° g abi d Gbéssé o ZONE Y T n Firou Borodarou 124 u Batia e Boukoubrou ouli A P B KONKWESSO CYNÉGÉTIQUE ' Ségbana L Gogounou MANDOURI DE LA Kérou Bagou Dassari Tanougou Nassoukou Sokotindji PENDJARI è Gouandé Cascades Brignamaro Libant ROFIA Tiélé Ede Tanougou I NAKI-EST Kédékou Sori Matéri D 513 ri Sota bo li vers DAPAONG R Monrou Tanguiéta A T A K O A A é E Guilmaro n O Toukountouna i KARENGI TI s Basso N è s u Gbéroubou Gnémasson a Î o u è è è É S k r T SANSANN - g Kouarfa o Gawézi GANDO Kobli A a r Gamia MANGO Datori m Kouandé é Dounkassa BABANA NAMONI H u u Manta o o Guéssébani -

Elevage Et Conservation Des Crocodiles » 13 Au 15 Novembre 2007 À La Tapoa, Parc Régional W Du Niger
Crédit photo : O. Born Actes du 1er Congrès d’Afrique de l’Ouest sur les Crocodiles « Elevage et Conservation des crocodiles » 13 au 15 novembre 2007 à la Tapoa, Parc Régional W du Niger Proceeding of 1st Workshop of the West African Countries on on Crocodilian farming and conservation 13-15 November 2007, La Tapoa Regional Parc W, Niger 1 Capture d‟un jeune crocodile du Nil dans la rivière Mékrou lors de la sortie de terrain réalisée par Hamissou Malam Garba, conservateur adjoint du parc et Magalie Péchaire coordinatrice de l‟organisation de ces premières rencontres. 2 Préface & Remerciements Forwords & Acknowledgments Le groupe des spécialistes des crocodiles de l‟UICN de la commission de sauvegarde des espèces (IUCN/SSC CSG) a été fondé en 1970 par un petit nombre de passionnés. Depuis cette date ce groupe s‟est réuni tous les 2 ans pour devenir aujourd‟hui, à l‟image de l‟UICN, un réseau de plus de 300 experts au service de la conservation des crocodiliens. En 2006, le congrès mondial du CSG s‟est réuni pour la première fois en France avec pour conséquence une présence accrue des représentants de pays francophones et plus particulièrement de nos amis d‟Afrique de l‟Ouest. A l‟issue de cette rencontre, Graham Webb, président du CSG et Richard Ferguson, Vice président pour l‟Afrique ont demandé à l‟équipe de la Ferme aux Crocodiles de parrainer les premières rencontres des pays d‟Afrique francophone sur le thème de l‟élevage et la conservation des crocodiles. Pour des raisons symboliques et pratiques, l‟hôtel de la Tapoa situé a proximité du point triple dans le parc régional du W à été retenu pour accueillir cette manifestation.