IN THIS ISSUE: a Focus on Pulmonary Transplantation Click on Titles Below to Jump to Article
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pursuit of Excellence Published for the Nursing Staff of Miami Children’S Hospital
PURSUIT OF EXCELLENCE PUBLISHED FOR THE NURSING STAFF OF MIAMI CHILDREN’S HOSPITAL Volume 7 • Issue 1 • Spring 2006 Miracles in Haiti By Carolyn Domina, ARNP, MSN, CNOR, CNABC Operating Room se Lea ur de ince 2003, a N r surgical team S from MCH has made multiple trips to Haiti to help children diagnosed with hydrocephalus. With the help of Project Medis- hare, Helping Hands Clinic and from the In t Pavillon Notre Dame th igh des Pauvres, where the e Spotl surgeries are performed in Haiti, we have been Debbie Salani able to make a differ- surgeries as possible before dark. When night Director of ence in the lives of dozens of families. falls, we generally lose power and water. Emergency Services The neurosurgical team has been per- The children that we treat range in age forming a procedure known as a ventricular from a few months to 12 years of age, with peritoneal shunt, which involves placement some being a bit older. Most of these kids Debbie brings to her of a small flexible plastic tube that diverts role more than 25 years excess cerebral spinal fluid from the brain continued on page 6 experience as a pediatric to another part of the body where the fluid nurse practitioner. can be reabsorbed. We also have performed a procedure known as an endoscopic third In this issue ventriculostomy (ETV) in which a small hole Degrees: is made in the floor of the third ventricle of Implementing a Humpty Dumpty FallsTM • Bachelor’s degree in the brain which allows the cerebrospinal fluid Scale & Prevention Program psychology from to bypass the obstruction and flow toward the A Day in the Life of a Nurse Marywood University in area where it can be excreted by the body. -

The Globalization of Chinese Food ANTHROPOLOGY of ASIA SERIES Series Editor: Grant Evans, University Ofhong Kong
The Globalization of Chinese Food ANTHROPOLOGY OF ASIA SERIES Series Editor: Grant Evans, University ofHong Kong Asia today is one ofthe most dynamic regions ofthe world. The previously predominant image of 'timeless peasants' has given way to the image of fast-paced business people, mass consumerism and high-rise urban conglomerations. Yet much discourse remains entrenched in the polarities of 'East vs. West', 'Tradition vs. Change'. This series hopes to provide a forum for anthropological studies which break with such polarities. It will publish titles dealing with cosmopolitanism, cultural identity, representa tions, arts and performance. The complexities of urban Asia, its elites, its political rituals, and its families will also be explored. Dangerous Blood, Refined Souls Death Rituals among the Chinese in Singapore Tong Chee Kiong Folk Art Potters ofJapan Beyond an Anthropology of Aesthetics Brian Moeran Hong Kong The Anthropology of a Chinese Metropolis Edited by Grant Evans and Maria Tam Anthropology and Colonialism in Asia and Oceania Jan van Bremen and Akitoshi Shimizu Japanese Bosses, Chinese Workers Power and Control in a Hong Kong Megastore WOng Heung wah The Legend ofthe Golden Boat Regulation, Trade and Traders in the Borderlands of Laos, Thailand, China and Burma Andrew walker Cultural Crisis and Social Memory Politics of the Past in the Thai World Edited by Shigeharu Tanabe and Charles R Keyes The Globalization of Chinese Food Edited by David Y. H. Wu and Sidney C. H. Cheung The Globalization of Chinese Food Edited by David Y. H. Wu and Sidney C. H. Cheung UNIVERSITY OF HAWAI'I PRESS HONOLULU Editorial Matter © 2002 David Y. -

List of Approved Food Items for Light Refreshment Restaurants
LIST OF APPROVED FOOD ITEMS FOR LIGHT REFRESHMENT RESTAURANTS Licensed light refreshment restaurants may only prepare and sell one of the following groups of food items for consumption on the premises – Group A (1) Noodles / vermicelli in soup with meat, offal, fish or sea food; (2) Wantun and dumplings in soup (also known as shui kau); (3) Boiled vegetables; (4) Tea, coffee, cocoa, any non-alcoholic drink or beverage made by adding water to prepared liquid or powder, and (5) Five self-specified snack items 【pre-prepared and supplied from approved / licensed sources, ready to eat after warming / reheating by electricity (excluding deep-frying and stir-frying )】*. or Group B (1) Rice congee with meat, offal, poultry, fish, sea food or frog; (2) Tea, coffee, cocoa, any non-alcoholic drink or beverage made by adding water to prepared liquid or powder; and (3) Five self-specified snack items 【pre-prepared and supplied from approved /licensed sources, ready to eat after warming / reheating by electricity (excluding deep-frying and stir-frying )】*. or Group C (any combination of the following seventeen items) (1) Bread, cakes and biscuits; (2) Toast including French toast; (3) Sandwiches; (4) Hot cakes, pancakes and waffles; (5) Oatmeal porridge and instant cereals; (6) Pastries (baking is not allowed but an electric warmer may be used to keep the pastries warm); (7) Eggs (boiled, poached, fried or scrambled); (8) Ham, bacon, western sausages, tinned meat and tinned fish; (9) Soup (prepared from tinned soup or powdered soup); (10) Macaroni -
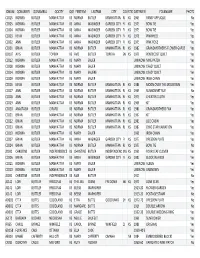
Quilt Project Index
IDNUM QONAMEFI QONAMELA QOCITY QOSTATEFIRSTNM LASTNM CITY COUNTYSTATEDATEMEN FOLKNAME PHOTOS CC015 NORMA BUTLER MANHATTAN KS NORMA BUTLER MANHATTAN RL KS 1965 PANSY APPLIQUE No CC005 NORMA BUTLER MANHATTAN KS ANNA WASINGER GARDEN CITY FI KS 1973 BOW TIE Yes CC004 NORMA BUTLER MANHATTAN KS ANNA WASINGER GARDEN CITY FI KS 1973 BOW TIE Yes CC003 KEVIN BUTLER MANHATTAN KS ANNA WASINGER GARDEN CITY FI KS 1978 PINWHEEL Yes CC002 ANN BUTLER MANHATTAN KS ANNA WASINGER GARDEN CITY FI KS 1975 PINK ROSE Yes CC001 BRIAN BUTLER MANHATTAN KS NORMA BUTLER MANHATTAN RL KS 1982 GRANDMOTHER'S FLOWER GARDE Yes DD107 AVIS BUTLER TOPEKA KS AVIS BUTLER TOPEKA SN KS 1979 PENTECOST QUILT Yes CC012 NORMA BUTLER MANHATTAN KS MARY SAUER UNKNOW NINE PATCH Yes CC008 NORMA BUTLER MANHATTAN KS MARY SAUER UNKNOW CRAZY QUILT Yes CC014 NORMA BUTLER MANHATTAN KS MARY SAUERE UNKNOW CRAZY QUILT Yes CC009 NORMA BUTLER MANHATTAN KS MARY SAUER UNKNOW IRISH CHAIN Yes CC016 KEVIN BUTLER MANHATTAN KS NORMA BUTLER MANHATTAN RL KS 1986 MOON OVER THE MOUNTAIN Yes CC017 ANN BUTLER MANHATTAN KS NORMA BUTLER MANHATTAN RL KS 1969 SUNBONNET SUE No CC018 ANN BUTLER MANHATTAN KS NORMA BUTLER MANHATTAN RL KS 1975 CHEATER CLOTH Yes CC019 ANN BUTLER MANHATTAN KS NORMA BUTLER MANHATTAN RL KS 1968 KIT Yes CC020 ANASTASIA BUTLER GIRARD KS NORMA BUTLER MANHATTAN RL KS 1980 GRANDMOTHERS FAN Yes CC021 BRIAN BUTLER MANHATTAN KS NORMA BUTLER MANHATTAN RL KS 1967 KIT Yes CC022 BRIAN BUTLER MANHATTAN KS NORMA BUTLER MANHATTAN RL KS 1983 LOG CABIN Yes CC023 BRIAN BUTLER MANHATTAN KS NORMA BUTLER MANHATTAN -

Math & Problem Solving Music & Art Reading, Words
Cut along the dotted line and place reference cards near your Early Literacy Station™ (ELS). *Title not included on ELS Tablet 1 2 Math & Problem Solving Music & Art Ages 1 - 3 Ages 1 - 3 • Fingertapps Jelly Jigsaw • Fingertapps Paint • Fingertapps Sky Writer • Getting Ready for Kindergarten • Getting Ready for Kindergarten • Giggles Kids My Musical World* • Giggles ABCs and 123s* • Giggles Kids Nursery Rhymes* • JumpStart Toddler • JumpStart Toddler • Millie’s Math House • Reader Rabbit Toddler • Reader Rabbit Toddler • Sesame Street Learn, Play & Grow • Sesame Street Learn, Play & Grow • Tux Paint • Trudy’s Time and Place House Early Literacy Station™ Version 12 Early Literacy Station™ Version 12 3 4 Reading, Words & Phonics -1 Reading, Words & Phonics - 2 Ages 1 - 3 Ages 1 - 3 • Bailey’s Book House • Little Red Riding Hood • Billy Goats Gruff • Playing with Sounds and Letters • Buckle My Shoe • Reader Rabbit Learn to Read with • Getting Ready for Kindergarten Phonics* • Giggles Kids Nursery Rhymes* • Reader Rabbit Toddler • Giggles ABCs and 123s* • Sammy’s Science House • Goldilocks and the Three Bears • Sesame Street Learn, Play & Grow • Harry and the Haunted House • The Berenstain Bears Get in a Fight • Humpty Dumpty • The Boy Who Cried Wolf • Jack and the Beanstalk • The Gingerbread Man • JumpStart Toddler • The Three Little Pigs • Little Monster at School Early Literacy Station™ Version 12 Early Literacy Station™ Version 12 Cut along the dotted line and place reference cards near your Early Literacy Station™ (ELS). *Title not included -

Product List Winter 2020/21 2 Winter 2020/21 Winter 2017 3
Product List Winter 2020/21 2 Winter 2020/21 Winter 2017 3 Contents Large and Small meals Large and Small Meals Textured Modified A huge selection of mains, soups, Savoury Pastries 04 Level 3 (Liquidised) 22 sides and desserts – created to Breakfast 04 Level 4 (Puréed) 22 sustain and nourish patients. Soups 05 Level 4 (Purée Petite) 25 Main meals – Meat Level 5 (Minced & Moist) 26 • Beef 06 Level 6 (Soft & Bite-Sized) 27 • Poultry 06 • Pork 08 • Other Meat 08 Special Diets • Lamb & Mutton 09 Gluten free 29 Main Meals - Fish 09 Free From 30 Main Meals - Vegetarian 10 Mini Meals Extra 31 Sides Energy dense 32 • Accompaniments 11 Low & Reduced Sugars Individual Desserts 32 • Vegetables 12 Finger Foods 33 • Potatoes and Rice 13 Desserts Ethnic Meals • Hot Desserts 14 Kosher Meals 35 • Cold Desserts 16 Kosher Desserts 35 • Cakes 16 Caribbean & West Indian Meals 36 Asian Halal Meals 37 CarteChoix Asian Halal Vegetarian Meals 38 Main meals – Meat • Beef 18 Dietary Codes 39 • Lamb 18 • Poultry 18 • Pork 18 Main Meals - Fish 18 Main Meals - Vegetarian 18 Main Meals - iWave Recommended 19 Desserts • Hot desserts 19 Making food that tastes great and aids patient recovery has been our mission from day one. It’s what our registered dietitian and team of qualified chefs work tirelessly for. Whatever a patient’s dietary needs, ethnic preference or taste, it’s about offering them something good to eat. Our Dietitian, Emily Stuart, is a healthcare expert as well as a member Minced Beef Hotpot : 324112 of the BDA 4 Winter 2020/21 Large and Small Meals Winter -

Department of Drama ® Presents
Paris Junior College Department of Drama ® presents Free Virtual Production on Zoom 7:30 p.m., Dec. 3, 4, 5 • 2:30 p.m., Dec. 6 Message from the Department of Drama Paris Junior College’s Department of Drama would like to ex- tend our warmest welcome to all in the 2020-21 theatre sea- son. Our Department has a tradition of exceptional students who have graced our stages, learned in our classrooms, and worked in our studios. As a student of theatre at PJC they are joining an accom- plished faculty of artists and scholars who are committed to creating, researching, and teaching exciting theatre and dra- ma. Our students develop skills both on and offstage while preparing to join the growing network of PJC Alumni in the professional and educational world of theatre arts. Whether students are a major, or just a curious student taking classes, we encourage them to become involved in the many activities of our department; this is an essential way to chal- lenge themselves and discover new insights as a part of their undergraduate education and experience. Students looking to venture into both music and drama have the option of a concentration in Musical Theatre here at Paris Junior College. On behalf of the students and faculty of the PJC Department of Drama we welcome you to our home. The Drama Faculty Alice in Cyberspace SCENES Scene 1: Alice’s Gaming Party Scene 2: The Kingdoms of the Red and White Queens Scene 3: Humpty Dumpty’s Wall Scene 4: The Lake of Tears with the Mock Turtle and Gryphon Scene 5: The Mad Tea Party Scene 5.5 / Scene -

Using Date Specific Searches on Google Books to Disconfirm Prior
social sciences $€ £ ¥ Article Using Date Specific Searches on Google Books to Disconfirm Prior Origination Knowledge Claims for Particular Terms, Words, and Names Mike Sutton and Mark D. Griffiths * ID Department of Social Sciences, Nottingham Trent University, 50 Shakespeare Street, Nottingham NG1 4FQ, UK; [email protected] * Correspondence: mark.griffi[email protected] Received: 2 March 2018; Accepted: 12 April 2018; Published: 16 April 2018 Abstract: Back in 2004, Google Inc. (Menlo Park, CA, USA) began digitizing full texts of magazines, journals, and books dating back centuries. At present, over 25 million books have been scanned and anyone can use the service (currently called Google Books) to search for materials free of charge (including academics of any discipline). All the books have been scanned, converted to text using optical character recognition and stored in its digital database. The present paper describes a very precise six-stage Boolean date-specific research method on Google, referred to as Internet Date Detection (IDD) for short. IDD can be used to examine countless alleged facts and myths in a systematic and verifiable way. Six examples of the IDD method in action are provided (the terms, words, and names ‘self-fulfilling prophecy’, ‘Humpty Dumpty’, ‘living fossil’, ‘moral panic’, ‘boredom’, and ‘selfish gene’) and each of these examples is shown to disconfirm widely accepted expert knowledge belief claims about their history of coinage, conception, and published origin. The paper also notes that Google’s autonomous deep learning AI program RankBrain has possibly caused the IDD method to no longer work so well, addresses how it might be recovered, and how such problems might be avoided in the future. -

Adventuring with Books: a Booklist for Pre-K-Grade 6. the NCTE Booklist
DOCUMENT RESUME ED 311 453 CS 212 097 AUTHOR Jett-Simpson, Mary, Ed. TITLE Adventuring with Books: A Booklist for Pre-K-Grade 6. Ninth Edition. The NCTE Booklist Series. INSTITUTION National Council of Teachers of English, Urbana, Ill. REPORT NO ISBN-0-8141-0078-3 PUB DATE 89 NOTE 570p.; Prepared by the Committee on the Elementary School Booklist of the National Council of Teachers of English. For earlier edition, see ED 264 588. AVAILABLE FROMNational Council of Teachers of English, 1111 Kenyon Rd., Urbana, IL 61801 (Stock No. 00783-3020; $12.95 member, $16.50 nonmember). PUB TYPE Books (010) -- Reference Materials - Bibliographies (131) EDRS PRICE MF02/PC23 Plus Postage. DESCRIPTORS Annotated Bibliographies; Art; Athletics; Biographies; *Books; *Childress Literature; Elementary Education; Fantasy; Fiction; Nonfiction; Poetry; Preschool Education; *Reading Materials; Recreational Reading; Sciences; Social Studies IDENTIFIERS Historical Fiction; *Trade Books ABSTRACT Intended to provide teachers with a list of recently published books recommended for children, this annotated booklist cites titles of children's trade books selected for their literary and artistic quality. The annotations in the booklist include a critical statement about each book as well as a brief description of the content, and--where appropriate--information about quality and composition of illustrations. Some 1,800 titles are included in this publication; they were selected from approximately 8,000 children's books published in the United States between 1985 and 1989 and are divided into the following categories: (1) books for babies and toddlers, (2) basic concept books, (3) wordless picture books, (4) language and reading, (5) poetry. (6) classics, (7) traditional literature, (8) fantasy,(9) science fiction, (10) contemporary realistic fiction, (11) historical fiction, (12) biography, (13) social studies, (14) science and mathematics, (15) fine arts, (16) crafts and hobbies, (17) sports and games, and (18) holidays. -

Tolerance Testing for Cooked Porridge Made from a Sorghum Based Fortified Blended Food
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by K-State Research Exchange TOLERANCE TESTING FOR COOKED PORRIDGE MADE FROM A SORGHUM BASED FORTIFIED BLENDED FOOD by SIRICHAT CHANADANG B.S., Chiang Mai University, 2009 A THESIS submitted in partial fulfillment of the requirements for the degree MASTER OF SCIENCE Department of Human Nutrition College of Human Ecology KANSAS STATE UNIVERSITY Manhattan, Kansas 2015 Approved by: Major Professor Dr. Kadri Koppel Abstract Products must be tolerant to many conditions, particularly when those products are prepared by consumers. Consumers may not measure added ingredients, they may add or leave out ingredients specified in recipes, or change cooking and holding times for foods. Fortified blended food (FBFs) are used as a source of nutrition for disaster or famine relief in developing countries and sorghum is looked at as a potential alternative to wheat and corn based products that are currently being used as FBFs. Porridge products are the most common dishes prepared from FBFs with a wide range of solids content, cooking times and variations in added ingredients such as sugar and fruit. This study was intended to evaluate the tolerance to preparation variations for a porridge product made as a FBF intended for food aid. Whole Sorghum Soy Blend (WSSB), a fortified, extruded, ground cooked cereal was selected as the FBF for this study. Descriptive sensory analysis was performed to evaluate the tolerance of porridge products made from variations in ingredients and cooking procedures. In this study, most sensory properties were only marginally affected by variations in ingredients or procedures. -

TRADITIONAL HIGH ANDEAN CUISINE ORGANISATIONS and RESCUING THEIR Communities
is cookbook is a collection of recipes shared by residents of High Andean regions of Peru STRENGTHENING HIGH ANDEAN INDIGENOUS and Ecuador that embody the varied diet and rich culinary traditions of their indigenous TRADITIONAL HIGH ANDEAN CUISINE ORGANISATIONS AND RESCUING THEIR communities. Readers will discover local approaches to preparing some of the unique TRADITIONAL PRODUCTS plants that the peoples of the region have cultivated over millennia, many of which have found international notoriety in recent decades including grains such as quinoa and amaranth, tubers like oca (New Zealand yam), olluco (earth gems), and yacon (Peruvian ground apple), and fruits such as aguaymanto (cape gooseberry). e book is the product of a broader effort to assist people of the region in reclaiming their agricultural and dietary traditions, and achieving both food security and viable household incomes. ose endeavors include the recovery of a wide variety of unique plant varieties and traditional farming techniques developed during many centuries in response to the unique environmental conditions of the high Andean plateau. TRADITIONAL Strengthening Indigenous Organizations and Support for the Recovery of Traditional Products in High-Andean zones of Peru and Ecuador HIGH ANDEAN Food and Agricultural Organization of the United Nations Regional Office for Latin America and the Caribbean CUISINE Av. Dag Hammarskjöld 3241, Vitacura, Santiago de Chile Telephone: (56-2) 29232100 - Fax: (56-2) 29232101 http://www.rlc.fao.org/es/proyectos/forsandino/ FORSANDINO STRENGTHENING HIGH ANDEAN INDIGENOUS ORGANISATIONS AND RESCUING THEIR TRADITIONAL PRODUCTS Llaqta Kallpanchaq Runa Kawsay P e r u E c u a d o r TRADITIONAL HIGH ANDEAN CUISINE Allin Mikuy / Sumak Mikuy Published by Food and Agriculture Organization of the United Nations Regional Office for Latin America and the Caribbean (FAO/RLC) FAO Regional Project GCP/RLA/163/NZE 1 Worldwide distribution of English edition Traditional High Andean Cuisine: Allin Mikuy / Sumak Mikuy FAORLC: 2013 222p.; 21x21 cm. -

Black Woman Black Man Spirit Tree
Cane River Chronicles I & II: A collection of poems/short stories birthed from the memories of my ancestors of Cane River (Natchitoches Parish) By Debra Roberson I. Black woman You are the creator of all life. In your womb is the foundation of a past that defines our future. From your being evolves all nations, kindred, and tongues. Black to Red to Yellow to White- we owe our existence to you. We behold your glory- innate, incomprehensible, intimate, you are beautiful beyond words. Knowledgeable beyond understanding. Your strength spans man’s weakness by creating a bridge from generation to generation. Without you we cease to be. For you, Black woman, are the essence of God given life itself. Black man You have toiled until your hands are calloused and bruised. You have had to stand by while your family was torn apart and your women raped. Even though from your loins flowed life, you were enslaved and told that you are less than human. You hung your head in shame as you passed a white person and you had to cross to the other side of the street. Your strong black back bore the pain and sorrow of your heritage and with every crack of that whip they tried to beat that pride out of you. You were born of kings and queens. You have forgotten that you are royalty. Where formal slavery hid in the shadows, you are still enslaved in the prisons of this “Great Country. “You matter” Don’t lose sight of your greatness. We need you, Black man.