Chapter I Basic Characteristics of Soils Outline
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Infiltration, Permeability, Liquid Limit and Plastic Limit of Soil (IJIRST/ Volume 4 / Issue 6 / 003)
IJIRST –International Journal for Innovative Research in Science & Technology| Volume 4 | Issue 6 | November 2017 ISSN (online): 2349-6010 Infiltration, Permeability, Liquid Limit and Plastic Limit of Soil S. Andavan Vegi Varun Assistant Professor Student Department of Civil Engineering Department of Civil Engineering Saveetha School of Engineering, Saveetha University, Saveetha School of Engineering, Saveetha University, Chennai. India Chennai. India Abstract Precipitation falling on the soil wets down and it starts penetrating into the soil. Water stores to the formal level the soil moisture deficiency excess moving down by the gravity force through percolation or seepage to build up the water table. The water is driven into the porous soil by force of gravity. First the water wets soil grain sand then the extra water moves down due to gravitational force. The rate at which a soil absorbing the water in a given time is called infiltration rate and it depends on soil characteristics such as hydraulic conductivity, soil structure, vegetation cover. The infiltration plays an important role in generation of runoff volume, if infiltration rate of given soil is less than intensity of rainfall then it results in either accumulation of water on soil surface or in runoff. The different soil conditions affect the soil infiltration rate. Compacted soils due to movement of agricultural machines have a low infiltration rate which is prone to runoff generation. Infiltration will be maximum at the beginning and it decays exponentially and gets a constant value. There will be a decrease in infiltration rate day by day due to the saturation of the soil where as on the first day the infiltration rate will be more because soil will be dry in condition. -

Hydraulic Conductivity of Gravelly Soils with Various Fine-Grained Content
Hydraulic Conductivity of Gravelly Soils with Various Fine-Grained Content Yi Zhou 1,2 and Zhenbin Peng1,2 1 School of Geosciences and Info-Physics, Central South University, Changsha, 410083, China 2 Key Laboratory of Metallogenic Prediction of Nonferrous Metals and Geological Environment Monitoring, Central South University, Changsha, 410083, China e-mail: [email protected] ABSTRACT Gravelly soils with a certain clay content has proven to be a good performance anti-seepage material. However, there are many factors affecting the permeability coefficient of gravel soil. For example, the content of fine particles, properties of fines and compactive effort. Laboratory experiments are conducted to study the effect of above factors. The hydraulic conductivities of soils with different fines, coarse grains and mix ratios are measured using the variable water head permeability tests. The test results show that the permeability coefficient of gravelly soils decreases linearly with the increase of fine particle content and then tends to be stable. The permeability coefficient of gravelly soil decreases with the increase of fine material liquid limit and plasticity index.The permeability coefficient of gravelly soils with clay as fine material decreases in the form of exponential function with the increase of compaction effort. KEYWORDS: Gravelly soil; Optimal moisture content; Hydraulic conductivity; Compactive effort INTRODUCTION Gravelly soils can solve the problems of low strength, easy settlement, deformation and cracking of [1-2] clay as impervious material due to its high density, strength and low compressibility after rolling . In [3-5] recent years, it has been widely used as anti-seepage material for core wall of earth-rockfill dam . -

Consolidation and Permeability Behavior of High Porosity Baltic Seabed Sediments
University of Rhode Island DigitalCommons@URI Open Access Master's Theses 1994 Consolidation and Permeability Behavior of High Porosity Baltic Seabed Sediments Ajoykumar Ag University of Rhode Island Follow this and additional works at: https://digitalcommons.uri.edu/theses Recommended Citation Ag, Ajoykumar, "Consolidation and Permeability Behavior of High Porosity Baltic Seabed Sediments" (1994). Open Access Master's Theses. Paper 916. https://digitalcommons.uri.edu/theses/916 This Thesis is brought to you for free and open access by DigitalCommons@URI. It has been accepted for inclusion in Open Access Master's Theses by an authorized administrator of DigitalCommons@URI. For more information, please contact [email protected]. CONSOLIDATION AND PERMEABILITY BEHAVIOR OF HIGH POROSITY BALTIC SEABED SEDIMENTS BY AJOYKUMAR AG A THESIS SUBMITrED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE IN OCEAN ENGINEERING UNIVERSITY OF RHODE ISLAND 1994 MASTER OF SCIENCE THESIS OF AJOYKUMAR AG APPROVED: Thesis Committee Major Professor_ __..,,...._....""""""'"""""""~"'"""""''----=--=----=--- UNIVERSITY OF RHODE ISLAND 1994 ABSTRACT The consolidation and permeability characteristics of high porosity surficial sediments from the Coastal Benthic Boundary Layer (Naval Research Laboratory) Special Research Program test site in the EckemfOrde Bay Baltic Sea were studied using a backpressured, constant rate of deformation (CRD) consolidation and permeability testing system driven by flow pumps The silty clay sediments from the Baltic test site are characterized by high void ratios (above 6), large organic content and dissolved or free methane gas bubbles. The high organic content (up to 17%) influences several of the unique features of these sediments including high water contents (up to 500%; porosities up to 93%), high consistency limits and high compressibility. -

Evaluation of Hydraulic Conductivity
EVALUATION OF HYDRAULIC CONDUCTIVITY METHODS USING LABORATORY, FIELD AND EMPIRICAL EQUATION – A CASE STUDY IN MOJO RIVER STREAM BED SADIK ABDULJELIL KEDIR MASTER OF SCIENCE ADDIS ABABA SCIENCE AND TECHNOLOGY UNIVERSITY FEBRUARY 2018 EVALUATION OF HYDRAULIC CONDUCTIVITY METHODS USING LABORATORY, FIELD AND EMPIRICAL EQUATION – A CASE STUDY IN MOJO RIVER STREAM BED By SADIK ABDULJELIL KEDIR A Thesis Submitted to The Department of Civil Engineering for the Partial Fulfilment of the Requirements for the Degree of Master of Science in Civil Engineering (Geotechnical Engineering) ADDIS ABABA SCIENCE AND TECHNOLOGY UNIVERSITY FEBRUARY 2018 Declaration I hereby declare that this thesis entitled “Evaluation of Hydraulic Conductivity Methods Using Laboratory, Field And Empirical Equation – A Case Study in Mojo River Stream Bed” was composed by myself, with the guidance of my advisor, that the work contained herein is my own except where explicitly stated otherwise in the text, and that this work has not been submitted, in whole or in part, for any other degree or processional qualification. Name: Signature, Date: ii Approval Page This Msc thesis Proposal entitled “Evaluation of Hydraulic Conductivity Methods Using Laboratory, Field And Empirical Equations – A Case Study in Mojo River Stream Bed” has been approved by the following examiners in partial fulfilment of the requirement for the degree of Master of Science in Geotechnical Engineering. Date of Defense: February 2, 2018 Principal Advisor 1. Dr. Argaw Asha ______________ ____________ Signature Date Members of the Examining board: 1) Dr. Daniel Teklu 28/05/2018 Principal Examiner Signature Date 2) Dr. Avinash M. Potdar _______________ _____________ Co. Examiner Signature Date 3) Dr. -

Hydraulic Conductivity in Layered Saturated Soils Assessed Through a Novel Physical Model
Hydraulic conductivity in layered saturated soils assessed through a novel physical model Eduardo Dulcey-Leal a, Fausto Molina-Gómez b & Lenin Alexander Bulla-Cruz c a Facultad de Estudios a Distancia, Universidad Militar Nueva Granada, Colombia. [email protected] b Facultad de Ingeniería, Universidad Militar Nueva Granada, Colombia. [email protected] c Facultad de Ingeniería, Universidad Nacional de Colombia, Colombia. [email protected] Received: April 27th, 2017. Received in revised form: November 1rd, 2017. Accepted: February15th, 2018. Abstract This paper introduces a novel physical model for measuring the hydraulic conductivity of granular materials in saturated conditions. An innovation in the location of the device’s piezometers allows the device to be used to assess the permeability of layered soils for both parallel and perpendicular flows. The model’s square section construction makes soil compaction easy. Design methodology took issues of construction, calibration and implementation into account. Permeability coefficients in directions parallel and perpendicular to soil stratification were measured, and Student’s t-test was performed for the relation of experimental results and existing numerical correlations. Analysis of the results shows that the physical model can replicate seepage in layered soils with parallel and perpendicular flows as occurs in field. Furthermore, it was found that it is possible to validate the experimental calibration data by use of statistical techniques. Keywords: equivalent permeability coefficient; seepage; statistical analysis; t-test. Estimación de la conductividad hidráulica en suelos saturados estratificados mediante un nuevo modelo físico Resumen Este documento presenta un modelo físico novedoso que permite medir la conductividad hidráulica en materiales granulares saturados. -

Simulation-Based Solutions Reducing Soil and Groundwater Contamination from Fertilizers in Arid and Semi-Arid Regions: Case Study the Eastern Nile Delta, Egypt
International Journal of Environmental Research and Public Health Article Simulation-Based Solutions Reducing Soil and Groundwater Contamination from Fertilizers in Arid and Semi-Arid Regions: Case Study the Eastern Nile Delta, Egypt Ismail Abd-Elaty 1, Lorenzo Pugliese 2 , Martina Zelenakova 3,* , Peter Mesaros 4 and Abdelaziz El Shinawi 5 1 Department of Water and Water Structures Engineering, Faculty of Engineering, Zagazig University, Zagazig 44519, Egypt; [email protected] 2 Department of Agroecology, Aarhus University, 8830 Tjele, Denmark; [email protected] 3 Department of Environmental Engineering, Faculty of Civil Engineering, Technical University of Kosice, 04200 Kosice, Slovakia 4 Department of Construction Technology and Management, Faculty of Civil Engineering, Technical University of Kosice, 04200 Kosice, Slovakia; [email protected] 5 Environmental Geophysics Lab (ZEGL), Geology Department, Faculty of Science, Zagazig University, Zagazig 44519, Egypt; [email protected] * Correspondence: [email protected] Received: 7 November 2020; Accepted: 12 December 2020; Published: 15 December 2020 Abstract: Intensive agriculture requires increasing application of fertilizers in order to sustain food production. Improper use of these substances in combination with increasing seawater intrusion results in long-term and nonpoint soil and groundwater contamination. In this work, a 3-D groundwater and solute transport numerical model was created to simulate the effect of excessive fertilizers application along the Bahr El Baqar drain system, in the eastern Nile Delta, Egypt. The geotechnical properties of the soils, hydrologic parameters, and unconfined compressive strength were determined at different sites and used as input parameters for the model. Model results showed that silty clay soils are able to contain the contaminations and preserve the groundwater quality. -
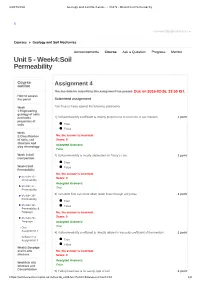
Unit 5 - Week4:Soil Permeability
24/07/2018 Geology and Soil Mechanics - - Unit 5 - Week4:Soil Permeability X [email protected] ▼ Courses » Geology and Soil Mechanics Announcements Course Ask a Question Progress Mentor Unit 5 - Week4:Soil Permeability Course outline Assignment 4 The due date for submitting this assignment has passed. Due on 2016-02-26, 23:55 IST. How to access the portal Submitted assignment Week Tick True or False against the following statements 1:Engineering geology of soils and Index 1) Soil permeability coefficient is directly proportional to void ratio of soil medium. 1 point properties of soils True False Week 2:Classification No, the answer is incorrect. of soils, soil Score: 0 structure and Accepted Answers: clay mineralogy False Week 3:Soil 2) Soil permeability is mostly dependent on Darcy’s Law. 1 point Compaction True Week4:Soil False Permeability No, the answer is incorrect. Module 16 - Score: 0 Permeability Accepted Answers: Module 17 - True Permeability 3) Turbulent flow can occur when water flows through soil pores. 1 point Module 18 - Permeability True Module 19 - False Permeability & Seepage No, the answer is incorrect. Module 20 - Score: 0 Seepage Accepted Answers: Quiz : True Assignment 4 4) Soil permeability coefficient is directly related to viscosity coefficient of the medium 1 point Solution For Assignment 4 True False Week5:Seepage and In-situ No, the answer is incorrect. stresses Score: 0 Accepted Answers: Week6:In situ stresses and False Consolidation 5) Falling head test is for sandy type of soil 1 point https://onlinecourses.nptel.ac.in/noc16_ce01/unit?unit=32&assessment=34 1/6 24/07/2018 Geology and Soil Mechanics - - Unit 5 - Week4:Soil Permeability Week True 7:Consolidation False No, the answer is incorrect. -

48. Variations in Sediment Physical Properties and Permeability of Mud-Volcano Deposits from Napoli Dome and Adjacent Mud Volcanoes1
Robertson, A.H.F., Emeis, K.-C., Richter, C., and Camerlenghi, A. (Eds.), 1998 Proceedings of the Ocean Drilling Program, Scientific Results, Vol. 160 48. VARIATIONS IN SEDIMENT PHYSICAL PROPERTIES AND PERMEABILITY OF MUD-VOLCANO DEPOSITS FROM NAPOLI DOME AND ADJACENT MUD VOLCANOES1 Achim Kopf,2 M. Ben Clennell,3 and Angelo Camerlenghi4 ABSTRACT Active mud volcanoes on the Mediterranean Ridge accretionary prism were sampled during Bannock Cruises 88 and 89 and Ocean Drilling Program Leg 160. Permeability tests on undisturbed whole-round samples from Napoli dome (Site 971) using a back-pressured system at effective stresses that ranged from 200 to 700 kPa revealed low hydraulic conductivities of the clay- rich, undisturbed “mud breccias,” which ranged from 5 × 10–8 to 5 × 10–9 mm/s. In general, conductivities of sediments from the footwall, flank, and crest of Napoli dome dropped to half their value when the load was incrementally increased from 500 up to 700 kPa. Pore volume, initially 50%−55%, was reduced to around 25% after multiple incremental loading between the permeability tests. Experiments on remolded mud breccia from Napoli dome (Site 971) and adjacent mud volcanoes using a geotechnical shear box as well as a Vane apparatus revealed peak shear strengths of 200−400 kPa. Plasticity indices also vary significantly for the mud-volcano deposits (i.e., from 25% to >40%), presumably because of variations in the composition and amount of the clay fraction. The friction angles obtained with shear tests indicate that montmorillonites (φ′ peak = 10°−15°) and fine grained carbonates (φ′ peak 27°−30°) are the two dominant mineral phases in the matrix. -

The Effective Porosity and Grain Size Relations in Permeability Functions
Discussion Paper | Discussion Paper | Discussion Paper | Discussion Paper | Hydrol. Earth Syst. Sci. Discuss., 11, 6675–6714, 2014 www.hydrol-earth-syst-sci-discuss.net/11/6675/2014/ doi:10.5194/hessd-11-6675-2014 © Author(s) 2014. CC Attribution 3.0 License. This discussion paper is/has been under review for the journal Hydrology and Earth System Sciences (HESS). Please refer to the corresponding final paper in HESS if available. The effective porosity and grain size relations in permeability functions K. Urumović1 and K. Urumović Sr.*, ** 1Croatian Geological Survey, Sachsova 2, P.O. Box 268, 10001 Zagreb, Croatia *formally at: University of Zagreb, Faculty of Mining, Geology and Petroleum Engineering, Zagreb, Croatia **retired Received: 5 April 2014 – Accepted: 21 May 2014 – Published: 24 June 2014 Correspondence to: K. Urumović ([email protected]) Published by Copernicus Publications on behalf of the European Geosciences Union. 6675 Discussion Paper | Discussion Paper | Discussion Paper | Discussion Paper | Abstract Hydrogeological parameters of coherent and incoherent deposits are deeply dependent of their granulometric characteristics. These relations were shaped in formulas and defaultly used for calculation of hydraulic conductivity, and are valid only 5 for uniform incoherent materials, mostly sands. In this paper, the results of analyses of permeability and specific surface area as a function of granulometric composition of various sediments – from siltey clays to very well graded gravels are presented. The effective porosity and the referential grain size are presented as fundamental granulometric parameters which express an effect of forces operating fluid movement 10 through the saturated porous media. Suggested procedures for calculating referential grain size and determining effective (flow) porosity result with parameters that reliably determine specific surface area and permeability. -

Correlation Between Laboratory and Field Permeability of Soils and Rocks
ﺒﺴﻡ ﺍﷲ ﺍﻟﺭﺤﻤﻥ ﺍﻟﺭﺤﻴﻡ University of Khartoum Faculty of Engineering & Architecture Correlation between Laboratory and Field Permeability of Soils and Rocks A Thesis Submitted in Partial Fulfillment of the Requirements For the Degree of Master of Science in Structural Engineering By: Ahmed Abdallah Eltayeb Elfaki June 2006 DEDICATION • To my family • To all people whom works in a tough weather ACKNOWLEDGEMENTS The author wishes to express his great indebtness to the supervisor Dr. Hussien Arabi, Building and Road Research Institute (BRRI), University of Khartoum, for his kind help and guidance. Considerable thanks are due to the staff of (BRRI) for their great assistance. Thanks are extended to the laboratory staff for their help. In addition, I would like to express my appreciation to my family for supporting me. Ahmed ABSTRACT Permeability affects the performance of structure through the soil and rock pavements. Percolation of water, through the interconnected voids of soil and rock pavement, causes a number of soil and rock engineering problems such as settlement of buildings, yield of wells, seepage through and below the earth structures. In this study, laboratory and field permeability tests, based on the principles of falling head and lugeon test, were conducted on different borehole in Merowe irrigation project area and Khartoum New International Airport (KNIA), to study the correlation between the laboratory and the field permeability values. The main objective was to assess whether the field permeability values could be estimated from laboratory permeability values or any other parameter estimated from laboratory or field. The results show that there was a significant difference between the laboratory permeability measured and the field permeability values. -

Predicting the Permeability of Sandy Soils From
PREDICTING THE PERMEABILITY OF SANDY SOILS FROM GRAIN SIZE DISTRIBUTIONS A thesis submitted to Kent State University in partial fulfillment of the requirements for the Degree of Master of Science by Emine Mercan Onur May, 2014 Thesis written by Emine Mercan Onur B.S., Middle East Technical University, 2009 M.S., Kent State University, 2014 Approved by ___________________________________, Advisor ___________________________________, Chair, Department of Geology ___________________________________, Dean, College of Arts and Sciences ii TABLE OF CONTENTS LIST OF FIGURES ........................................................................................................... vi LIST OF TABLES ........................................................................................................... xiii ACKNOWLEDGEMENT ............................................................................................... xiv ABSTRACT ........................................................................................................................ 1 CHAPTER 1: INTRODUCTION ....................................................................................... 3 1.1 Background ............................................................................................................. 3 1.2 Factors affecting Permeability ................................................................................ 4 1.2.1 Effect of Grain Size and Grain Size Distribution ...................................................... 5 1.2.2 Effect of Density and -

Plasticity, Strength and Permeability of Reclaimed Asphalt Pavement and Lateritic Soil Blends I.I
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Covenant University Repository International Journal of Scientific & Engineering Research, Volume 5, Issue 6, June-2014 631 ISSN 2229-5518 Plasticity, Strength and Permeability of Reclaimed Asphalt Pavement and Lateritic Soil Blends I.I. Akinwumi Abstract— This paper presents the results of laboratory evaluation of the effects of the addition of reclaimed asphalt pavement (RAP), to an A-2 lateritic soil, on the plasticity, strength and permeability of the soil. The natural soil was classified as A-2-6(1), according to AASHTO classification system. RAP was added to the soil in 0, 4, 8 and 12%, by dry weight of the soil. Specific gravity, Atterberg limits, compaction, California bearing ratio (CBR), unconfined compression and permeability tests were conducted on each of the soil-RAP blends. Results obtained show that as RAP content in the blend increased, the plasticity index, optimum moisture content, maximum dry unit weight, swell potential, unconfined compressive strength and permeability decreased while the specific gravity, soaked and unsoaked California bearing ratios increased. These results indicate that RAP effectively improved, especially, the plasticity and permeability of the soil. It also indicates that deformation should be a major design criterion while planning the use of lateritic soil-RAP blend as a road pavement layer material. Index Terms— laterite, recycled asphalt, soil modification, subgrade, sub-base, tropical soil, unbound granular materials —————————— —————————— 1 INTRODUCTION N today’s world, issues of reusing and recycling of non- virgin aggregates gave more satisfactory results than wholly I renewable resources as a means of minimizing waste and using RAP.