Water Quality Annual Report 2010-2011-Aurangabad
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Annexure-V State/Circle Wise List of Post Offices Modernised/Upgraded
State/Circle wise list of Post Offices modernised/upgraded for Automatic Teller Machine (ATM) Annexure-V Sl No. State/UT Circle Office Regional Office Divisional Office Name of Operational Post Office ATMs Pin 1 Andhra Pradesh ANDHRA PRADESH VIJAYAWADA PRAKASAM Addanki SO 523201 2 Andhra Pradesh ANDHRA PRADESH KURNOOL KURNOOL Adoni H.O 518301 3 Andhra Pradesh ANDHRA PRADESH VISAKHAPATNAM AMALAPURAM Amalapuram H.O 533201 4 Andhra Pradesh ANDHRA PRADESH KURNOOL ANANTAPUR Anantapur H.O 515001 5 Andhra Pradesh ANDHRA PRADESH Vijayawada Machilipatnam Avanigadda H.O 521121 6 Andhra Pradesh ANDHRA PRADESH VIJAYAWADA TENALI Bapatla H.O 522101 7 Andhra Pradesh ANDHRA PRADESH Vijayawada Bhimavaram Bhimavaram H.O 534201 8 Andhra Pradesh ANDHRA PRADESH VIJAYAWADA VIJAYAWADA Buckinghampet H.O 520002 9 Andhra Pradesh ANDHRA PRADESH KURNOOL TIRUPATI Chandragiri H.O 517101 10 Andhra Pradesh ANDHRA PRADESH Vijayawada Prakasam Chirala H.O 523155 11 Andhra Pradesh ANDHRA PRADESH KURNOOL CHITTOOR Chittoor H.O 517001 12 Andhra Pradesh ANDHRA PRADESH KURNOOL CUDDAPAH Cuddapah H.O 516001 13 Andhra Pradesh ANDHRA PRADESH VISAKHAPATNAM VISAKHAPATNAM Dabagardens S.O 530020 14 Andhra Pradesh ANDHRA PRADESH KURNOOL HINDUPUR Dharmavaram H.O 515671 15 Andhra Pradesh ANDHRA PRADESH VIJAYAWADA ELURU Eluru H.O 534001 16 Andhra Pradesh ANDHRA PRADESH Vijayawada Gudivada Gudivada H.O 521301 17 Andhra Pradesh ANDHRA PRADESH Vijayawada Gudur Gudur H.O 524101 18 Andhra Pradesh ANDHRA PRADESH KURNOOL ANANTAPUR Guntakal H.O 515801 19 Andhra Pradesh ANDHRA PRADESH VIJAYAWADA -

Pre-Feasibility Report For
Pre-Feasibility Report For M/s Brahmagavhan Lift Irrigation Scheme- III, Near old Lakhampur Village, Tal. Gangapur, Dist. Aurangabad (MH) By Minor Irrigation Division No.1, Sinchan Bhavan Area, Jalna Road, Aurangabad (MH) Prepared By sd engineering services pvt. ltd. Aurangabad, MS {Environment Consultant} NABET Certificate No. NABET/EIA/1619/SA062 Contact No.: - 0240-2333622/09960634559 Email: - [email protected] [email protected] July 2020 Index Chapter No. Contents Page No. 1. Executive Summary 1 2. Introduction of the Project/ Background Information 2.1 Identification of the Project and Project Proponent 2 2.2 Brief Description of nature of the project 2 2.3 Need of project with description for region and country 2 2.4 Demand-Supply Gap 2 2.5 Imports vs. Indigenous Production 2 2.6 Export Possibility 3 2.7 Domestic / Export Markets 3 2.8 Employment Generation (Direct and Indirect) due to the project 3 3. Project Description 3.1 Type of Project including interlinked and interdependent project, 4 if any. 3.2 Location (map showing general location, specific location, and 4 project boundary & project site layout) with coordinates. 3.3 Details of alternative sites, considered and the basis of selecting 4 the proposed site particularly the environmental considerations gone into should be highlighted. 3.4 Size & magnitude of operations 5 3.5 Project description with process details (a schematic 5 diagram/flow chart showing the project layout, components of the project etc) should be given. 3.6 Raw material required along with estimated quantity, likely 11 source, marketing area of final products, mode of transport of raw material and finished products. -

District Ahmednagar
LIST OF PERMANENT SSI REGISTERED UNITS - DISTRICT AHMEDNAGAR. REGN REGN_ NAME OF THE UNIT ADR1 ADR2 MAJ_ACT_NAME1 PROD_NAME1 DIR_UNIT_NA DIR_UNI DIR_ADR DIR_ NO. YEAR ME1 T_NAME 1 ADR 2 2 M/S. CTS SA LEELAVATI NO.26/2 VE SHARAT , DI, M/S. POLICE AHMEDNA STATIO GAR PIPES. N, M/S.SATISH 29,CHA ,AH INDUSTRIE HURAN ME S, A DN M/S.SHRIR CS,NO. O.3 AM 1290,A- SH BOREWELL 1H.NO. RIR M/S.KARTIK S.NO.2 AD COPUTERS 74,/A10- E . 15,PLO MA M/S.AGRA ANANT EDI, WAL NIWAS, A`N INDUSTRIE MAHAV AG SHREE 5834 MARBLE TILAK ROAD MAHARASH NISAR TRA I.KURE FURNITUR SHI,H.N M/S. H.NO.1 TAL TRIMURTI 90, .SH ICE CAND MALWA RIG FAST-FAB 1101 DIS ENGINEERI KALKAI T NG WORKS SHRIG AH M/S.YOGAN 204,NI N,A DHARA WARA `NA FOOD TALKIE GA M/S. S.NO.1 GO SAIKRIPA 01/1+2, ND VEET RANJA AW NEW MOULA AH SUCCESS NA ME ENGINEERI AZAD DN FIBREX 107 ME INDUSTRIE BDATR DN S. ANGE AG AJID CHAHU AH HANSRAJ RANA ME KABNAWAT BK DN MANOHAR 5159 AH ENGINEERI MAHAT ME NG WORKS MA DN 3841 1997 BASE ACADEMY OF GALA NO. 65, MARKET M/S.BASE DUCAT GALA G COMPUTER YARD, SHOPING, ACADEMY ION. NO- CE AHMEDNAGAR OF 65,MAR NT KAMDHENU G.NO.1 POULTRY 0,BOND FARM RE,TAL- M/S. CST AH S.P.MUTHA NO.368 ME & 0, DN M/S. C-2/9, CH SHREYAS ANAND OW PRODUCTS NAGAR K, Page 1 DIC AHMEDNAGAR FRAME LIST M/S.VIMAL P.NO.1 PTA PLASTIC. -

Examining Climate Change Impact on the Variability of Ground Water Level: a Case Study of Ahmednagar District, India
J. Earth Syst. Sci. (2019) 128:122 c Indian Academy of Sciences https://doi.org/10.1007/s12040-019-1172-z Examining climate change impact on the variability of ground water level: A case study of Ahmednagar district, India N A Sivarajan, Anoop Kumar Mishra*, Mohammd Rafiq, V Nagraju and Sagarika Chandra Centre for Remote Sensing and Geoinformatics, Sathyabama Institute of Science and Technology, Chennai 600 119, India. *Corresponding author. e-mail: [email protected] MS received 8 May 2018; revised 10 December 2018; accepted 11 December 2018; published online 8 May 2019 This study focuses on examination of variability in the depth to water below ground level (bgl) from 1996 to 2016 in Ahmednagar district of Maharashtra, India in changing climate. Spatial variability of groundwater depth was mapped and classified as shallow, normal, deep and moderate using GIS modeling. Groundwater decline rates during successive decades were examined and critical areas with a significant fall in groundwater levels (GWL) were detected. Present study also focuses on examination of block-wise variability of GWL. Ground water level shows significant variability during 1996–2016 at confidence level 95%. The long term ground water level fluctuation indicates that water levels in major parts of the study area have declined drastically. The depth to ground water in major parts of the study area ranges between 6 and 15 m bgl except in Parner and Shrirampur blocks. Furthermore, depth to water level is >15 mbgl over Sangamner block during the drought year 2004. Present study reports a significant decadal increase of about 0.7 m in water leval decline over the study area. -
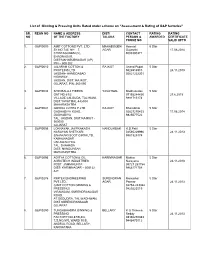
List of Ginning & Pressing Units Rated Under Scheme on “Assessment
List of Ginning & Pressing Units Rated under scheme on “Assessment & Rating of G&P factories” SR. REGN NO NAME & ADDRESS DIST/ CONTACT RATING RATING NO OF THE FACTORY TALUKA PERSON & AWARDED CERTIFICATE PHONE NO VALID UPTO 1. G&P/0009 AMIT COTTONS PVT. LTD MAHABOOBN Hemant 5 Star SY.NO.745, NH – 7, AGAR Gujarathi 17.08.2014 CHINTAGUDEM (V), 9000300371 EHADNAGAR, DIST:MAHABUBNAGAR (AP) PIN – 509 202 2. G&P/0010 JALARAM COTTON & RAJKOT Anand Popat 5 Star PROTEINS LTD 9426914910 24.11.2013 JASDAN- AHMEDABAD 02821222201 HIGHWAY, JASDAN, DIST: RAJKOT, GUJARAT, PIN: 360 050 3. G&P/0034 SHRI BALAJI FIBERS YAVATMAL Madhusudan 5 Star GAT NO:61/2 07153244430 27.6.2015 VILLAGE LALGUDA, TAL:WANI, 9881715174 DIST:YAVATMAL-445304 MAHARASHTRA 4. G&P/0041 GIRIRAJ COTEX P.LTD RAJKOT Bharatbhai 5 Star GADHADIYA ROAD, 02827270453 17.08.2014 GADHADIYA 9825077522 TAL: JASDAN, DIST;RAJKOT - 360050 GUJARAT 5. G&P/0056 LOKNAYAK JAYPRAKASH NANDURBAR R.D.Patil 5 Star NARAYAN SHETKARI 02565229996 24.11.2013 SAHAKARI SOOT GIRNI LTD, 9881925174 KAMALNAGAR UNTAWAD HOL TAL. SHAHADA DIST: NANDURBAR MAHARASHTRA 6. G&P/0096 ADITYA COTTON & OIL KARIMNAGAR Mukka 5 Star AGROTECH INDUSTRIES Narayana 24.11.2013 POST: JAMMIKUNTA 08727 253754 DIST: KARIMNAGAR – 505122 9866171754 A.P. 7. G&P/027 6 RIMTEX ENGINEERING SURENDRAN Manubhai 5 Star PVT.LTD., AGAR Parmar 24.11.2013 (UNIT COTTON GINNING & 02752-243322 PRESSING) 9825223519 VIRAMGAM, SURENDRANAGAR ROAD, AT.DEDUDRA, TAL.WADHWAN, DIST SURENDRANAGAR GUJARAT 8. G&P/0290 TUNGABHADRA GINNING & BELLARY K G Thimma 5 Star PRESSING Reddy 24.11.2013 FACTORY,NO.87/B,3/4, 08392250383 T.S.NO.970, WARD 10 B, 9448470112 ANDRAL ROAD, BELLARY, KARNATAKA 9. -

DISTRICT SURVEY REPORT for AHMEDNAGAR DISTRICT PART -A
DISTRICT SURVEY REPORT For AHMEDNAGAR DISTRICT PART -A FOR SAND MINING OR RIVER BED MINING 1 1.0 INTRODUCTION 1.1. LOCATION & GEOGRAPHICAL DATA: Ahmednagar is the largest district of Maharashtra State in respect of area, popularly known as “Nagar”. It is situated in the central part of the State in upper Godavari basin and partly in the Bhima basin and lies between north latitudes 18°19’ and 19°59’ and east longitudes 73°37’ and 75°32’ and falls in parts of Survey of India degree sheets 47 E, 47 I, 47 M, 47 J and 47 N. It is bounded by Nashik district in the north, Aurangabad and Beed districts to the east, Osmanabad and Solapur districts to the south and Pune and Thane districts to the west. The district has a geographical area of 17114 sq. km., which is 5.54% of the total State area. The district is well connected with capital City Mumbai & major cities in Maharashtra by Road and Railway. As per the land use details (2011), the district has an area of 134 sq. km. occupied by forest. The gross cultivable area of district is 15097 sq.km,whereas net area sown is 11463 sq.km. Figure 1 :Ahmednagar District Location Map 2 Table 1.1 – Geographical Data SSNo Geographical Data Unit Statistics . 18°19’ N and 19°59’N 1. Latitude and Longitude Degree To 73°37’E and 75°32’E 2. Geographical Area Sq. Km 17114 1.2. ADMINISTRATIVE SET UP: It is divided in to 14 talukas namely Ahmednagar, Rahuri, Shrirampur, Nevasa, Shevgaon, Pathardi, Jamkhed, Karjat, Srigonda, Parner, Akole, Sangamner, Kopargaon and Rahata. -

Gabriel India Limited Pune-Nashik Highway, Village Kuruli, Tai : Khed, GABRIEL Dist
29th Milestone, Gabriel India Limited Pune-Nashik Highway, Village Kuruli, Tai : Khed, GABRIEL Dist. Pune - 410 501 (India) Tel. :+ 91 - 2135 - 610700, 610757 Fax :+ 91 - 2135 - 610787, 610796 www.anandgroupindia.com July 22, 2019 BSE Limited National Stock Exchange of India Limited 25th Floor, P. J. Towers, Exchange Plaza, Sandra Kurla Complex, Dalal Street, Sandra (E), MUMBAI -400 001 MUMBAI - 400 051 (Company Code: 505714) (Company Code: GABRIEL) Sub: Newspaper Advertisement for Notice of 57th Annual General Meeting, Remote E voting and Book closure Dear Sirs, Pursuant to the provisions of Regulation 30 of SEBI (LODR) Regulations, 2015, please find attached a copy of advertisement regarding completion of dispatch of Notice of 57th Annual General Meeting of the Company and details of remote e-voting and Book closure, published in the Business standard and Loksatta on July 20, 2019. We request you to take the above information on record and kindly acknowledge the receipt. Thanking you, Yours faithfully, For Ga~ Limited Nilesh Jain Company Secretary Encl: a/a Email id: [email protected] Registered Office · 29th Milestone, Pune-Nashik Highway, Village Kuruli, Ta.Iuka Khed, Dist. Pune - 410 501 (India) www .gabrielindia.com AIVAl'llD >> GIN- L34101PN1961PLC015735 k ~ 'k ~s rl Business Standard PUNE 20 /21 JULY.2019 13 MID Corporate Branch ® TRANSFORMERS & ELECTRICALS KERALA LIMITED 3rd Floor, Bharti Vidyapeeth Bhavan, LBS Marg, Navipeth, Pune NOTICE INVITING OPEN TENDERS (RE-TENDER) Phone No.: (020-24324981), Email: [email protected] -

Maharashtra State Boatd of Sec & H.Sec Education Pune
MAHARASHTRA STATE BOATD OF SEC & H.SEC EDUCATION PUNE PAGE : 1 College wise performance ofFresh Regular candidates for HSC MARCH-2019 Candidates passed College No. Name of the collegeStream Candidates Candidates Total Pass Registerd Appeared Pass UDISE No. Distin- Grade Grade Pass Percent ction I II Grade 11.01.001 ANNASAHEB AWATE COLLLAGE MANCHAR DIST PUNE SCIENCE 156 156 0 25 116 8 149 95.51 27250102212 ARTS 252 252 2 42 126 25 195 77.38 COMMERCE 209 209 36 118 51 0 205 98.08 TOTAL 617 617 38 185 293 33 549 88.97 11.01.002 M G JUNIOR COLLAGE, MANCHER, DIST PUNE SCIENCE 238 238 16 81 139 2 238 100.00 27250102207 TOTAL 238 238 16 81 139 2 238 100.00 11.01.003 JANATA VIDYAMANDIR JR OL OF COM,GHODEGAON SCIENCE 137 137 1 43 91 0 135 98.54 27250105704 AMBEGAON ARTS 121 121 0 19 70 7 96 79.33 COMMERCE 221 221 14 70 122 11 217 98.19 TOTAL 479 479 15 132 283 18 448 93.52 11.01.004 SHRI. SHIVAJI JUNIOR COL, DHAMANI AMBEGAON SCIENCE 44 44 0 1 29 6 36 81.81 27250100702 TOTAL 44 44 0 1 29 6 36 81.81 11.01.005 SHRI BHAIRAVNATH JR COL OF COM AVSARI KD SCIENCE 18 18 0 2 11 2 15 83.33 27250103503 AMBEGAON HSC.VOC 60 60 1 24 23 0 48 80.00 TOTAL 78 78 1 26 34 2 63 80.76 11.01.006 SHRI.BHAIRAVNATH VIDYADHAM HIGHER ARTS 32 32 0 5 19 1 25 78.12 27250105504 SEC.SCH,AMBEGAON COMMERCE 30 30 3 13 13 0 29 96.66 MAHARASHTRA STATE BOATD OF SEC & H.SEC EDUCATION PUNE PAGE : 2 College wise performance ofFresh Regular candidates for HSC MARCH-2019 Candidates passed College No. -

Ahmadnagar District Maharashtra
1836/BDR/2014 भारत सरकार जल संसाधन मंत्रालय के न्द्रीय भूममजल बो셍 ड GOVT OF INDIA MINISTRY OF WATER RESOURCES CENTRAL GROUND WATER BOARD महाराष्ट्र रा煍य के अंत셍डत अहमदन셍र जजले की भूजल विज्ञान जानकारी GROUND WATER INFORMATION AHMADNAGAR DISTRICT MAHARASHTRA By 饍िारा Vijesh V. K. विजेश िी. के . Scientist-B िैज्ञाननक ख मध्य क्षेत्र, ना셍पुर CENTRAL REGION NAGPUR 2014 AHMADNAGAR DISTRICT AT A GLANCE 1. GENERAL INFORMATION Geographical Area 17114 sq. km Administrative Divisions : Taluka- 14; Ahmednagar, Rahuri , (As on 31/03/2011) Shrirampur, Nevasa, Shevgaon, Pathardi, Jamkhed, Karjat, Srigonda, Parner, Akola, Sangamner, Kopargaon and Rahata. Villages : 1581 Population (2001) : 4,543,159 Average Annual Rainfall : 501.8 mm 2. GEOMORPHOLOGY Major Physiographic unit : 4; Hill and Ghat section; Foothill zone; Plateau and Plains Major Drainage : 2; Godavari and Bhima 3. LAND USE (2001-02) Forest Area : 1634 sq. km. Net Area Sown : 15097 sq. km. Cultivable Area : 11463 sq. km. 4. PRINCIPAL CROPS (2011) Rabi Sorghum : 5072 sq. km. Bajra : 3622 sq. km. Wheat : 1382 sq. km. Pearlmillet : 1282 sq. km. 5. IRRIGATION BY DIFFERENT SOURCES (2006-2007) Nos./Potential Created (ha) Dugwells : 176608 /446465 Tubewells/Borewells : 20320/ 54383 Surface flow schemes : 4374/8688 Surface lift schemes : 2432/7041 Total potential created in the district : 516576 7. GROUND WATER MONITORING WELLS (As on 31/05/2011) Dugwells : 26 Piezometers : 9 8. GEOLOGY Recent : Alluvium Upper Cretaceous-Lower Eocene : Deccan Trap Basalt 9. HYDROGEOLOGY Water Bearing Formation : Hard Rock: Deccan Traps- Weathered/Fractured/Jointed massive or vesicular Basalt Soft Rock: Alluvium- Sand and gravel. -

List of Rated Ginning & Pressing Units
LIST OF STAR RATED GINNING & PRESSING FACTORIES REGN NO RATING SR. CONTACT PERSON WITH NAME & ADDRESS OF THE FACTORY DIST/TALUKA AWARDED NO. PHONE NO # ANDHRA PRADESH G&P/0763 SRI KAILASANADHA COTTON SYNDICATE (P) LTD., ADMN OFF: DOOR NO.5-87-60/2, 5TH LINE, CHANDRAMOULI NAGAR, T.P.KRISHNA RAO FIVE STAR 1 GUNTUR GUNTUR-522 007 9246730999 FACTORY:VELURU ROAD, GANAPAVARAM, GUNTUR (DT.) AP G&P/0949 RAAGHAVENDRA SPIN COT MILLS D.NO.213/D, CHINTHAPALLIPADU ROAD, PURNCHANDRA RAO 2 GUNTUR FOUR STAR KURNUTHALA VILLAGE, GUNTUR DIST., 9246485137 PIN - 522017 AP G&P/0951 GAYATRI COTTON MILLS PULLADIGUNTA VILLAGE, I. BASAVAIAH 3 NEAR M. LAXMAIAH WOMEN'S ENGG. GUNTUR 0863 2286123 FOUR STAR COLLEGE (OPP) 0940263425 GUNTUR DIST. PIN-522017 AP G&P/0514 VIJAYA CHAITANYA ENTERPRISES PRIVATE LIMITED DHULIPALLA - VILLAGE SUBBA RAO UNNAVA 4 SATTENAPALLI - MANDAL GUNTUR 08641 - 214477 FOUR STAR GUNTUR DISTRICT, PIN - 522412 9440652788 ANDRA PRADESH G&P/0487 SRI VIJAYA VENKATESWARA COTTON MILLS PVT.LTD D.NO.105 D, DOKIPARRU VILLAGE VISADALA(POST), MEDIKONDUR M.RAJASEKHARA RAO MANDAL, GUNTUR-522 438 0863 2290553 5 GUNTUR THREE STAR CORR: 2C, JAYA VIJAYA RESIDENCY 08632290552 LAKSHMIPURAM 4TH LANE 09440261361 GUNTUR-522 007(T.S.) G&P/0515 LAKSHMI COTTON TRADING CO. PVS SANKAR NEAR N.S.CANAL, 6 GUNTUR 0863 - 2235039 THREE STAR SATTENAPALLI - 522 403 9440621515 GUNTUR DISTRICT. AP G&P/0516 NSL TEXTILES LIMITED (FORMERLY PRABHAT INDUSTRIAL CORPORATION LTD.) P.RAJASEKHARA RAO 7 GINNING & PRESSING UNIT GUNTUR 9396418611 THREE STAR MADUGULA ROAD, GURAJALA - 522415 9581412219 GUNTUR (DIST.) ANDRA PRADESH G&P/0519 NSL TEXTILES (EDLAPADU)LTD. -

A Case Study of Newasa, Shani Shingnapur and Shirdi
PILGRIMAGES, TOURISM AND THE NEED OF ENGLISH: A CASE STUDY OF NEWASA, SHANI SHINGNAPUR AND SHIRDI RAVINDRA G. VAIDYA Assistant Professor & Head Department of English New Atrs, Commerce and Science College, Shevgaon Dist- Ahmednagar, (MS), India Language has been a primary medium of communication and a carrier of information. Being multilingual and multicultural nation variety of dialects is in use in the different regions of India. In the era of globalization cultural exchange across the borders through foreign visits has become a common phenomenon. Multilingual pattern of the country however, is a major barrier in this cultural dialogue. According to a famous proverb, language in the country changes at the distance of every five kilometers. Based on the linguistic pattern, most of the Indians are bilingual. Most of them are competent in their respective mother tongue and Hindi which is one of the official languages of India. Nationally acknowledged holy places such as Newasa, Shani Shingnapur and Shirdi, located in the Ahmednagar district of Maharashtra are endowed with great religious and cultural heritage. These places have witnessed growing number of foreign devotees in the last decade. They are naturally very eager to know the cultural and religious importance of the holy places. However, the lack of common medium of communication leaves them disappointed. The present paper explores the opportunities that local graduates of Newasa, Shani Shingnapur and Shirdi could have had they been fluent speakers of English. It also focuses on the prospects of employment that language can bring to the doorsteps of the graduates who have acquired the basic skills of the English language. -

District Br Code Branch Name Ifsc Code Branch
DISTRICT BR_CODE BRANCH NAME IFSC CODE BRANCH ADDRESS PHONE_NO CITY_DESC MICR_CODE EMAIL_ID AHMEDNAGAR 0670 AHMEDNAGAR CBIN0280670 NEAR LAXMIBAI KARANJA , 2345895 SHEVGAON 414016001 [email protected] AHMEDNAGAR, DISTAHMEDNAGAR MAHARASHTRA, PIN-414001 AHMEDNAGAR 0671 SANGAMNER (MAHARASTRA) CBIN0280671 J.M. LOHATI BHAVAN, MAIN 225483 NASIK 422016892 [email protected] ROADSANGAMNER, DIST. AHMEDNAGAR (MAHARASTRA) , PIN- 422605 AHMEDNAGAR 0672 SHRIGONDA CBIN0280672 VIJAY CHOWK , TAL: SHRIGONDA, KARMALA 416016285 [email protected] DIST: AHMEDNAGAR, PIN-413701 AHMEDNAGAR 0673 SHRIRAMPUR (MAHARASHTRA) CBIN0280673 KHATOD'S BUILDING CITY SURVEY KARMALA 413016890 [email protected] NO.970(A) , NEAR SANGAMNER RDSHRIRAMPUR , DIST: AHMED NAGAR (MAHARASTRA), PIN-413709 AHMEDNAGAR 0674 SHRI SHIVAJINAGAR CBIN0280674 POST RAHURI FACTORY , TAL: RAHURI KARMALA 416016486 [email protected] , DIST: AHMEDNAGAR, PIN-413706 AHMEDNAGAR 0675 GANESHNAGAR CBIN0280675 P.O. RANJANGAON KHURD , SHRI KARMALA 413016542 [email protected] GANESH SAHAKARI KARKHANA , BLDG.GANESHNAGARTAL:KOPARGAO N , PIN-413719 AHMEDNAGAR 0676 NEWASA CBIN0280676 GANDHI BHAWAN , TAL: NEWASA P.B. SHEVGAON 414016397 [email protected] NO. 6, DIST: AHMEDNAGAR, PIN- 414603 AHMEDNAGAR 0677 VAMBORI CBIN0280677 RATHI BUILDINGBANK ROAD , KARMALA 413016425 [email protected] VAMBORI TALRAHURI , DISTAHMEDNAGAR, PIN-413704 AHMEDNAGAR 0990 SAHAJANANDNAGAR CBIN0280990 SAHAKARI SAKHAR KARKHANA BLDG., NANDGAON (DIST.423016431