A Study of the Temporal and Spatial Distribution of Ichthyoplankton And
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Disease List for Aquaculture Health Certificate
Quarantine Standard for Designated Species of Imported/Exported Aquatic Animals [Attached Table] 4. Listed Diseases & Quarantine Standard for Designated Species Listed disease designated species standard Common name Disease Pathogen 1. Epizootic haematopoietic Epizootic Perca fluviatilis Redfin perch necrosis(EHN) haematopoietic Oncorhynchus mykiss Rainbow trout necrosis virus(EHNV) Macquaria australasica Macquarie perch Bidyanus bidyanus Silver perch Gambusia affinis Mosquito fish Galaxias olidus Mountain galaxias Negative Maccullochella peelii Murray cod Salmo salar Atlantic salmon Ameirus melas Black bullhead Esox lucius Pike 2. Spring viraemia of Spring viraemia of Cyprinus carpio Common carp carp, (SVC) carp virus(SVCV) Grass carp, Ctenopharyngodon idella white amur Hypophthalmichthys molitrix Silver carp Hypophthalmichthys nobilis Bighead carp Carassius carassius Crucian carp Carassius auratus Goldfish Tinca tinca Tench Sheatfish, Silurus glanis European catfish, wels Negative Leuciscus idus Orfe Rutilus rutilus Roach Danio rerio Zebrafish Esox lucius Northern pike Poecilia reticulata Guppy Lepomis gibbosus Pumpkinseed Oncorhynchus mykiss Rainbow trout Abramis brama Freshwater bream Notemigonus cysoleucas Golden shiner 3.Viral haemorrhagic Viral haemorrhagic Oncorhynchus spp. Pacific salmon septicaemia(VHS) septicaemia Oncorhynchus mykiss Rainbow trout virus(VHSV) Gadus macrocephalus Pacific cod Aulorhynchus flavidus Tubesnout Cymatogaster aggregata Shiner perch Ammodytes hexapterus Pacific sandlance Merluccius productus Pacific -
Schedule Onlinepdf
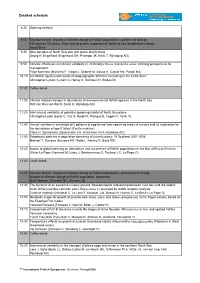
Detailed schedule 8:30 Opening session 9:00 Keynote lecture: Impacts of climate change on flatfish populations - patterns of change 100 days to 100 years: Short and long-term responses of flatfish to sea temperature change David Sims 9:30 Nine decades of North Sea sole and plaice distributions Georg H. Engelhard (Engelhard GH, Pinnegar JK, Kell LT, Rijnsdorp AD) 9:50 Climatic effects on recruitment variability in Platichthys flesus and Solea solea: defining perspectives for management. Filipe Martinho (Martinho F, Viegas I, Dolbeth M, Sousa H, Cabral HN, Pardal MA) 10:10 Are flatfish species with southern biogeographic affinities increasing in the Celtic Sea? Christopher Lynam (Lynam C, Harlay X, Gerritsen H, Stokes D) 10:30 Coffee break 11:00 Climate related changes in abundance of non-commercial flatfish species in the North Sea Ralf van Hal (van Hal R, Smits K, Rijnsdorp AD) 11:20 Inter-annual variability of potential spawning habitat of North Sea plaice Christophe Loots (Loots C, Vaz S, Koubii P, Planque B, Coppin F, Verin Y) 11:40 Annual variation in simulated drift patterns of egg/larvae from spawning areas to nursery and its implication for the abundance of age-0 turbot (Psetta maxima) Claus R. Sparrevohn (Sparrevohn CR, Hinrichsen H-H, Rijnsdorp AD) 12:00 Broadscale patterns in population dynamics of juvenile plaice: W Scotland 2001-2008 Michael T. Burrows (Burrows MT, Robb L, Harvey R, Batty RS) 12:20 Impact of global warming on abundance and occurrence of flatfish populations in the Bay of Biscay (France) Olivier Le Pape (Hermant -

Early Stages of Fishes in the Western North Atlantic Ocean Volume
ISBN 0-9689167-4-x Early Stages of Fishes in the Western North Atlantic Ocean (Davis Strait, Southern Greenland and Flemish Cap to Cape Hatteras) Volume One Acipenseriformes through Syngnathiformes Michael P. Fahay ii Early Stages of Fishes in the Western North Atlantic Ocean iii Dedication This monograph is dedicated to those highly skilled larval fish illustrators whose talents and efforts have greatly facilitated the study of fish ontogeny. The works of many of those fine illustrators grace these pages. iv Early Stages of Fishes in the Western North Atlantic Ocean v Preface The contents of this monograph are a revision and update of an earlier atlas describing the eggs and larvae of western Atlantic marine fishes occurring between the Scotian Shelf and Cape Hatteras, North Carolina (Fahay, 1983). The three-fold increase in the total num- ber of species covered in the current compilation is the result of both a larger study area and a recent increase in published ontogenetic studies of fishes by many authors and students of the morphology of early stages of marine fishes. It is a tribute to the efforts of those authors that the ontogeny of greater than 70% of species known from the western North Atlantic Ocean is now well described. Michael Fahay 241 Sabino Road West Bath, Maine 04530 U.S.A. vi Acknowledgements I greatly appreciate the help provided by a number of very knowledgeable friends and colleagues dur- ing the preparation of this monograph. Jon Hare undertook a painstakingly critical review of the entire monograph, corrected omissions, inconsistencies, and errors of fact, and made suggestions which markedly improved its organization and presentation. -

Hairtail and Frostfish (Trichiuridae) Exploitation Status Undefined
I & I NSW WILD FISHERIES RESEARCH PROGRAM Hairtail and Frostfish (Trichiuridae) EXPLOITATION STATUS UNDEFINED No local biological information available for either species in this group, but growth and maturity have been studied for Trichiurus lepturus from the East China Sea, where it supports a major fishery. SCIENTIFIC NAME STANDARD NAME COMMENT Trichiurus lepturus largehead hairtail Lepidopus caudatus frostfish Trichiurus lepturus Image © Bernard Yau Background The hairtail is commonly around 100 cm in length and about 2 kg in weight but reaches a The largehead hairtail (Trichiurus lepturus) maximum length of about 220 cm and weight belongs to the family Trichiuridae which, of 3.5 kg. worldwide, includes nine genera and about 30 species generally referred to as cutlassfishes or Overseas studies have observed that adults scabbardfishes. Off NSW, at least four species feed at the surface during the day, and retreat of trichiurids are found in deepwater, but the to deeper waters at night. In contrast, juveniles most well known member of the family to most and small adults tend to feed at night at the people is the hairtail, found in shallow coastal surface, and aggregate into schools at depths waters and estuaries. during the day. The adult hairtail diet consists mainly of fish with occasional squid and A cosmopolitan species, the largehead hairtail crustaceans, whereas juveniles mainly feed on is subject to significant fisheries off many planktonic crustaceans, euphausiids and small Asian countries, particularly China and Korea. fish. The world catch reportedly now exceeds 1.5 million t annually. In eastern Australia, Reported landings in NSW generally range hairtail occasionally school in coastal bays between 10 and 25 t with catches greatest and estuaries where they may be targeted during March-May. -

Investigating the Biological and Socio-Economic Impacts of Marine
Investigating the biological and socio-economic impacts of marine protected area network design in Europe Submitted by Kristian Metcalfe January 2013 to the Durrell Institute of Conservation and Ecology, School of Anthropology and Conservation, University of Kent as a thesis for the degree of Doctor of Philosophy in Biodiversity Management SUPERVISORS Dr Robert J Smith - Durrell Institute of Conservation and Ecology (DICE) Dr Sandrine Vaz - Institut Français de Recherche pour l‟exploitation de la Mer (IFREMER) Professor Stuart R Harrop - Durrell Institute of Conservation and Ecology (DICE) ii ABSTRACT Marine ecosystems are under increasing pressure from a diverse range of threats. Many national governments have responded to these threats by establishing marine protected area (MPA) networks. One such approach for designing MPA networks is systematic conservation planning, which is now considered the most effective system for designing protected area networks. However, the main exception to this trend is Europe, where the designation of MPAs is still largely based on expert opinion, despite growing awareness that these existing methods are not the most effective. Therefore, there is a need to demonstrate how systematic conservation planning can be used to inform MPA design in European waters and show how this approach can fit within existing marine conservation policy and practice. This thesis brings together a range of biological, legal and socio-economic data to address these issues and is comprised of four main chapters: After the introductory chapter, this thesis begins with a review of how existing approaches for guiding the selection of MPAs in Europe compare to conservation planning best practice (Chapter 2). -

Diversidade Funcional Da Ictiofauna Da Zona De Arrebentação De Jaguaribe (...)
FAVERO, FLT. Diversidade funcional da ictiofauna da zona de arrebentação de Jaguaribe (...) UNIVERSIDADE FEDERAL RURAL DE PERNAMBUCO PRÓ-REITORIA DE PESQUISA E PÓS-GRADUAÇÃO PROGRAMA DE PÓS-GRADUAÇÃO EM RECURSOS PESQUEIROS E AQUICULTURA DIVERSIDADE FUNCIONAL DA ICTIOFAUNA DA ZONA DE ARREBENTAÇÃO DE JAGUARIBE, ITAMARACÁ, LITORAL NORTE DE PERNAMBUCO Fernanda de Lima Toledo Favero Dissertação apresentada ao Programa de Pós-Graduação em Recursos Pesqueiros e Aquicultura da Universidade Federal Rural de Pernambuco, como exigência para obtenção do título de Mestre. Prof. Dr. WILLIAM SEVERI Orientador Recife, Agosto/ 2019 FAVERO, FLT. Diversidade funcional da ictiofauna da zona de arrebentação de Jaguaribe (...) 2 Dados Internacionais de Catalogação na Publicação (CIP) Sistema Integrado de Bibliotecas da UFRPE Biblioteca Central, Recife-PE, Brasil F273d Favero, Fernanda de Lima Toledo. Diversidade funcional da ictiofauna da zona de arrebentação de Jaguaribe, Itamaracá, litoral norte de Pernambuco / Fernanda de Lima Toledo Favero. – Recife, 2019. 66 f.: il. Orientador(a): William Severi. Dissertação (Mestrado) – Universidade Federal Rural de Pernambuco, Programa de Pós-Graduação em Recursos Pesqueiros e Aquicultura, Recife, BR-PE, 2019. Inclui referências. 1. Guildas funcionais 2. Ecomorfologia 3. Índices de diversidade funcional I. Severi, William, orient. II. Título. CDD 639.3 FAVERO, FLT. Diversidade funcional da ictiofauna da zona de arrebentação de Jaguaribe (...) 3 UNIVERSIDADE FEDERAL RURAL DE PERNAMBUCO PRÓ-REITORIA DE PESQUISA E PÓS-GRADUAÇÃO PROGRAMA DE PÓS-GRADUAÇÃO EM RECURSOS PESQUEIROS E AQÜICULTURA DIVERSIDADE FUNCIONAL DA ICTIOFAUNA DA ZONA DE ARREBENTAÇÃO DE JAGUARIBE, ITAMARACÁ, LITORAL NORTE DE PERNAMBUCO Fernanda de Lima Toledo Favero Dissertação julgada adequada para obtenção do título de mestre em Recursos Pesqueiros e Aquicultura. -

Fishes of Terengganu East Coast of Malay Peninsula, Malaysia Ii Iii
i Fishes of Terengganu East coast of Malay Peninsula, Malaysia ii iii Edited by Mizuki Matsunuma, Hiroyuki Motomura, Keiichi Matsuura, Noor Azhar M. Shazili and Mohd Azmi Ambak Photographed by Masatoshi Meguro and Mizuki Matsunuma iv Copy Right © 2011 by the National Museum of Nature and Science, Universiti Malaysia Terengganu and Kagoshima University Museum All rights reserved. No part of this publication may be reproduced or transmitted in any form or by any means without prior written permission from the publisher. Copyrights of the specimen photographs are held by the Kagoshima Uni- versity Museum. For bibliographic purposes this book should be cited as follows: Matsunuma, M., H. Motomura, K. Matsuura, N. A. M. Shazili and M. A. Ambak (eds.). 2011 (Nov.). Fishes of Terengganu – east coast of Malay Peninsula, Malaysia. National Museum of Nature and Science, Universiti Malaysia Terengganu and Kagoshima University Museum, ix + 251 pages. ISBN 978-4-87803-036-9 Corresponding editor: Hiroyuki Motomura (e-mail: [email protected]) v Preface Tropical seas in Southeast Asian countries are well known for their rich fish diversity found in various environments such as beautiful coral reefs, mud flats, sandy beaches, mangroves, and estuaries around river mouths. The South China Sea is a major water body containing a large and diverse fish fauna. However, many areas of the South China Sea, particularly in Malaysia and Vietnam, have been poorly studied in terms of fish taxonomy and diversity. Local fish scientists and students have frequently faced difficulty when try- ing to identify fishes in their home countries. During the International Training Program of the Japan Society for Promotion of Science (ITP of JSPS), two graduate students of Kagoshima University, Mr. -

An Annotated Bibliography of Diet Studies of Fish of the Southeast United States and Gray’S Reef National Marine Sanctuary
Marine Sanctuaries Conservation Series MSD-05-2 An annotated bibliography of diet studies of fish of the southeast United States and Gray’s Reef National Marine Sanctuary U.S. Department of Commerce February 2005 National Oceanic and Atmospheric Administration National Ocean Service Office of Ocean and Coastal Resource Management Marine Sanctuaries Division About the Marine Sanctuaries Conservation Series The National Oceanic and Atmospheric Administration’s Marine Sanctuary Division (MSD) administers the National Marine Sanctuary Program. Its mission is to identify, designate, protect and manage the ecological, recreational, research, educational, historical, and aesthetic resources and qualities of nationally significant coastal and marine areas. The existing marine sanctuaries differ widely in their natural and historical resources and include nearshore and open ocean areas ranging in size from less than one to over 5,000 square miles. Protected habitats include rocky coasts, kelp forests, coral reefs, sea grass beds, estuarine habitats, hard and soft bottom habitats, segments of whale migration routes, and shipwrecks. Because of considerable differences in settings, resources, and threats, each marine sanctuary has a tailored management plan. Conservation, education, research, monitoring and enforcement programs vary accordingly. The integration of these programs is fundamental to marine protected area management. The Marine Sanctuaries Conservation Series reflects and supports this integration by providing a forum for publication and discussion of the complex issues currently facing the National Marine Sanctuary Program. Topics of published reports vary substantially and may include descriptions of educational programs, discussions on resource management issues, and results of scientific research and monitoring projects. The series facilitates integration of natural sciences, socioeconomic and cultural sciences, education, and policy development to accomplish the diverse needs of NOAA’s resource protection mandate. -

Updated Checklist of Marine Fishes (Chordata: Craniata) from Portugal and the Proposed Extension of the Portuguese Continental Shelf
European Journal of Taxonomy 73: 1-73 ISSN 2118-9773 http://dx.doi.org/10.5852/ejt.2014.73 www.europeanjournaloftaxonomy.eu 2014 · Carneiro M. et al. This work is licensed under a Creative Commons Attribution 3.0 License. Monograph urn:lsid:zoobank.org:pub:9A5F217D-8E7B-448A-9CAB-2CCC9CC6F857 Updated checklist of marine fishes (Chordata: Craniata) from Portugal and the proposed extension of the Portuguese continental shelf Miguel CARNEIRO1,5, Rogélia MARTINS2,6, Monica LANDI*,3,7 & Filipe O. COSTA4,8 1,2 DIV-RP (Modelling and Management Fishery Resources Division), Instituto Português do Mar e da Atmosfera, Av. Brasilia 1449-006 Lisboa, Portugal. E-mail: [email protected], [email protected] 3,4 CBMA (Centre of Molecular and Environmental Biology), Department of Biology, University of Minho, Campus de Gualtar, 4710-057 Braga, Portugal. E-mail: [email protected], [email protected] * corresponding author: [email protected] 5 urn:lsid:zoobank.org:author:90A98A50-327E-4648-9DCE-75709C7A2472 6 urn:lsid:zoobank.org:author:1EB6DE00-9E91-407C-B7C4-34F31F29FD88 7 urn:lsid:zoobank.org:author:6D3AC760-77F2-4CFA-B5C7-665CB07F4CEB 8 urn:lsid:zoobank.org:author:48E53CF3-71C8-403C-BECD-10B20B3C15B4 Abstract. The study of the Portuguese marine ichthyofauna has a long historical tradition, rooted back in the 18th Century. Here we present an annotated checklist of the marine fishes from Portuguese waters, including the area encompassed by the proposed extension of the Portuguese continental shelf and the Economic Exclusive Zone (EEZ). The list is based on historical literature records and taxon occurrence data obtained from natural history collections, together with new revisions and occurrences. -

Checklist of Marine Demersal Fishes Captured by the Pair Trawl Fisheries in Southern (RJ-SC) Brazil
Biota Neotropica 19(1): e20170432, 2019 www.scielo.br/bn ISSN 1676-0611 (online edition) Inventory Checklist of marine demersal fishes captured by the pair trawl fisheries in Southern (RJ-SC) Brazil Matheus Marcos Rotundo1,2,3,4 , Evandro Severino-Rodrigues2, Walter Barrella4,5, Miguel Petrere Jun- ior3 & Milena Ramires4,5 1Universidade Santa Cecilia, Acervo Zoológico, R. Oswaldo Cruz, 266, CEP11045-907, Santos, SP, Brasil 2Instituto de Pesca, Programa de Pós-graduação em Aquicultura e Pesca, Santos, SP, Brasil 3Universidade Federal de São Carlos, Programa de Pós-Graduação em Planejamento e Uso de Recursos Renováveis, Rodovia João Leme dos Santos, Km 110, CEP 18052-780, Sorocaba, SP, Brasil 4Universidade Santa Cecília, Programa de Pós-Graduação de Auditoria Ambiental, R. Oswaldo Cruz, 266, CEP11045-907, Santos, SP, Brasil 5Universidade Santa Cecília, Programa de Pós-Graduação em Sustentabilidade de Ecossistemas Costeiros e Marinhos, R. Oswaldo Cruz, 266, CEP11045-907, Santos, SP, Brasil *Corresponding author: Matheus Marcos Rotundo: [email protected] ROTUNDO, M.M., SEVERINO-RODRIGUES, E., BARRELLA, W., PETRERE JUNIOR, M., RAMIRES, M. Checklist of marine demersal fishes captured by the pair trawl fisheries in Southern (RJ-SC) Brazil. Biota Neotropica. 19(1): e20170432. http://dx.doi.org/10.1590/1676-0611-BN-2017-0432 Abstract: Demersal fishery resources are abundant on continental shelves, on the tropical and subtropical coasts, making up a significant part of the marine environment. Marine demersal fishery resources are captured by various fishing methods, often unsustainably, which has led to the depletion of their stocks. In order to inventory the marine demersal ichthyofauna on the Southern Brazilian coast, as well as their conservation status and distribution, this study analyzed the composition and frequency of occurrence of fish captured by pair trawling in 117 fishery fleet landings based in the State of São Paulo between 2005 and 2012. -

Gonadal Structure and Gametogenesis of Trigla Lyra
Zoological Studies 41(4): 412-420 (2002) Gonadal Structure and Gametogenesis of Trigla lyra (Pisces: Triglidae) Marta Muñoz*, Maria Sàbat, Sandra Mallol and Margarida Casadevall Biologia Animal, Departament Ciències Ambientals, Universitat de Girona, Campus de Montilivi, Girona 17071, Spain. (Accepted July 19, 2002) Marta Muñoz, Maria Sàbat, Sandra Mallol and Margarida Casadevall (2002) Gonadal structure and gameto- genesis of Trigla lyra (Pisces: Triglidae). Zoological Studies 41(4): 412-420. The gonadal structure and devel- opment stages of germ cells of Trigla lyra are described for both sexes. The ovigerous lamellae of the saccular cystovarian ovaries spread from the periphery to the center of the gonad where the lumen is located. In addi- tion to the postovulatory follicles and atretic oocytes, seven stages of development are described based on the histological and ultrastructural characteristics of the oocytes. Gonadal development is of the “synchronous group” type. This kind of development and the presence of postovulatory follicles in ovaries which still contain developing vitellogenic oocytes suggest that T. lyra spawns on multiple occasions over a relatively prolonged period of time. Drops of oil in the mature oocyte indicate that spawning is pelagic. The testes are lobular and of the “unrestricted spermatogonial” type. Male germ cells are divided into 8 stages that were principally ana- lyzed by means of transmission electron microscopy. The morphological characteristics of the spermatozoon, as a short head and a barely differentiated -

A List of Common and Scientific Names of Fishes from the United States And
t a AMERICAN FISHERIES SOCIETY QL 614 .A43 V.2 .A 4-3 AMERICAN FISHERIES SOCIETY Special Publication No. 2 A List of Common and Scientific Names of Fishes -^ ru from the United States m CD and Canada (SECOND EDITION) A/^Ssrf>* '-^\ —---^ Report of the Committee on Names of Fishes, Presented at the Ei^ty-ninth Annual Meeting, Clearwater, Florida, September 16-18, 1959 Reeve M. Bailey, Chairman Ernest A. Lachner, C. C. Lindsey, C. Richard Robins Phil M. Roedel, W. B. Scott, Loren P. Woods Ann Arbor, Michigan • 1960 Copies of this publication may be purchased for $1.00 each (paper cover) or $2.00 (cloth cover). Orders, accompanied by remittance payable to the American Fisheries Society, should be addressed to E. A. Seaman, Secretary-Treasurer, American Fisheries Society, Box 483, McLean, Virginia. Copyright 1960 American Fisheries Society Printed by Waverly Press, Inc. Baltimore, Maryland lutroduction This second list of the names of fishes of The shore fishes from Greenland, eastern the United States and Canada is not sim- Canada and the United States, and the ply a reprinting with corrections, but con- northern Gulf of Mexico to the mouth of stitutes a major revision and enlargement. the Rio Grande are included, but those The earlier list, published in 1948 as Special from Iceland, Bermuda, the Bahamas, Cuba Publication No. 1 of the American Fisheries and the other West Indian islands, and Society, has been widely used and has Mexico are excluded unless they occur also contributed substantially toward its goal of in the region covered. In the Pacific, the achieving uniformity and avoiding confusion area treated includes that part of the conti- in nomenclature.