Life Linked to Ice Final Draft
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

C S a S S C C S
C S A S S C C S Canadian Science Advisory Secretariat Secrétariat canadien de consultation scientifique Research Document 2010/066 Document de recherche 2010/066 Ecosystem status and trends report: Rapport de l’état des écosystèmes et Arctic Marine Ecozones des tendances : écozones marines de l’Arctique Andrea Niemi, Joclyn Paulic and Don Cobb Fisheries and Oceans Canada / Pêches et Océans Canada Central & Arctic Region / Région du Centre et de l’Arctique 501 University Crescent / 501, University Crescent Winnipeg, MB R3T 2N6 This series documents the scientific basis for the La présente série documente les fondements evaluation of aquatic resources and ecosystems scientifiques des évaluations des ressources et in Canada. As such, it addresses the issues of des écosystèmes aquatiques du Canada. Elle the day in the time frames required and the traite des problèmes courants selon les documents it contains are not intended as échéanciers dictés. Les documents qu’elle definitive statements on the subjects addressed contient ne doivent pas être considérés comme but rather as progress reports on ongoing des énoncés définitifs sur les sujets traités, mais investigations. plutôt comme des rapports d’étape sur les études en cours. Research documents are produced in the official Les documents de recherche sont publiés dans language in which they are provided to the la langue officielle utilisée dans le manuscrit Secretariat. envoyé au Secrétariat. This document is available on the Internet at: Ce document est disponible sur l’Internet à: http://www.dfo-mpo.gc.ca/csas/ ISSN 1499-3848 (Printed / Imprimé) ISSN 1919-5044 (Online / En ligne) © Her Majesty the Queen in Right of Canada, 2010 © Sa Majesté la Reine du Chef du Canada, 2010 TABLE OF CONTENTS / TABLE DES MATIÈRES ABSTRACT................................................................................................................................ -

Department of Environment– Wildlife Division
Department of Environment– Wildlife Division Wildlife Research Section Department of Environment Box 209 Igloolik, NU X0A 0L0 Tel: (867) 934-2179 Fax: (867) 934-2190 Email: [email protected] Frequently Asked Questions Government of Nunavut 1. What is the role of the GN in issuing wildlife research permits? On June 1, 1999, Nunavut became Canada’s newest territory. Since its creation, interest in studying its natural resources has steadily risen. Human demands on animals and plants can leave them vulnerable, and wildlife research permits allow the Department to keep records of what, and how much research is going on in Nunavut, and to use this as a tool to assist in the conservation of its resources. The four primary purposes of research in Nunavut are: a. To help ensure that communities are informed of scientific research in and around their communities; b. To maintain a centralized knowledgebase of research activities in Nunavut; c. To ensure that there are no conflicting or competing research activities in Nunavut; and d. To ensure that wildlife research activities abide by various laws and regulations governing the treatment and management of wildlife and wildlife habitat in Nunavut. 2. How is this process supported by the Nunavut Land Claims Agreement? Conservation: Article 5.1.5 The principles of conservation are: a. the maintenance of the natural balance of ecological systems within the Nunavut Settlement Area; b. the protection of wildlife habitat; c. the maintenance of vital, healthy, wildlife populations capable of sustaining harvesting needs as defined in this article; and d. the restoration and revitalization of depleted populations of wildlife and wildlife habitat. -
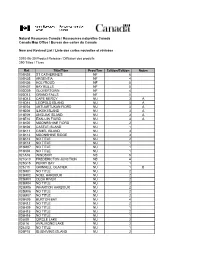
20100630 CMO Maps
Natural Resources Canada / Ressources naturelles Canada Canada Map Office / Bureau des cartes du Canada New and Revised List / Liste des cartes nouvelles et révisées 2010-06-30 Product Release / Diffusion des produits 290 Titles / Titres Ref. Title/Titre Prov/Terr Edition/Édition Notes 001N03 ST CATHERINE'S NF 6 001N05 ARGENTIA NF 4 001N06 HOLYROOD NF 5 001N07 BAY BULLS NF 5 002D09 GLOVERTOWN NF 4 002D13 GRAND FALLS NF 5 016D13 CAPE MERCY NU 3 A 016D14 LEOPOLD ISLAND NU 3 A 016E04 AKTIJARTUKAN FIORD NU 3 A 016E06 ILIKOK ISLAND NU 3 A 016E09 ANGIJAK ISLAND NU 3 A 016E10 EXALUIN FIORD NU 3 A 016K05 MOONESHINE FIORD NU 2 016K06 CASTLE ISLAND NU 1 016K11 CAMEL ISLAND NU 3 016K12 MOONSHINE RIDGE NU 3 016K13 NO TITLE NU 2 016K14 NO TITLE NU 1 016M07 NO TITLE NU 1 016N04 NO TITLE NU 1 021A16 WINDSOR NS 5 021G10 FREDERICTON JUNCTION NB 4 025G15 PERRY BAY NU 1 025J10 GRINNELL GLACIER NU 1 B 025M01 NO TITLE NU 2 025M02 NOEL HARBOUR NU 2 025M03 OLGA RIVER NU 2 025M04 NO TITLE NU 2 025M05 WHARTON HARBOUR NU 2 025M06 NO TITLE NU 2 025M07 NO TITLE NU 2 025N09 BURTON BAY NU 4 025N12 NO TITLE NU 2 026H09 NO TITLE NU 1 026H15 NO TITLE NU 2 026H16 NO TITLE NU 1 026I09 CIRCLE LAKE NU 2 026I16 AVALIKONG LAKE NU 2 026J02 NO TITLE NU 1 026P15 IDJUNIVING ISLAND NU 2 027B01 NO TITLE NU 2 C 027B02 NO TITLE NU 2 C 027B03 NO TITLE NU 2 C 027B04 NO TITLE NU 2 C 027B14 NO TITLE NU 1 027D05 NO TITLE NU 1 027D06 IITIDLIALUK LAKE NU 1 027D12 NO TITLE NU 1 027D13 NO TITLE NU 1 031E01 WILBERFORCE ON 6 031E02 HALIBURTON ON 6 034N13 LONG REACH ISLAND NU 2 C 036A05 JUBILEE -

Article Is Available On- 2012
The Cryosphere, 14, 2673–2686, 2020 https://doi.org/10.5194/tc-14-2673-2020 © Author(s) 2020. This work is distributed under the Creative Commons Attribution 4.0 License. Clouds damp the radiative impacts of polar sea ice loss Ramdane Alkama1, Patrick C. Taylor2, Lorea Garcia-San Martin1, Herve Douville3, Gregory Duveiller1, Giovanni Forzieri1, Didier Swingedouw4, and Alessandro Cescatti1 1European Commission – Joint Research Centre, Via Enrico Fermi, 2749, 21027 Ispra (VA), Italy 2NASA Langley Research Center, Hampton, Virginia, USA 3Centre National de Recherches Météorologiques, Météo-France/CNRS, Toulouse, France 4EPOC, Université de Bordeaux, Allée Geoffroy Saint-Hilaire, Pessac 33615, France Correspondence: Ramdane Alkama ([email protected]) and Patrick C. Taylor ([email protected]) Received: 19 November 2019 – Discussion started: 19 December 2019 Revised: 19 June 2020 – Accepted: 6 July 202 – Published: 21 August 2020 Abstract. Clouds play an important role in the climate sys- 1 Introduction tem: (1) cooling Earth by reflecting incoming sunlight to space and (2) warming Earth by reducing thermal energy loss to space. Cloud radiative effects are especially important Solar radiation is the primary energy source for the Earth in polar regions and have the potential to significantly alter system and provides the energy driving motions in the atmo- the impact of sea ice decline on the surface radiation budget. sphere and ocean, the energy behind water phase changes, Using CERES (Clouds and the Earth’s Radiant Energy Sys- and the energy stored in fossil fuels. Only a fraction (Loeb tem) data and 32 CMIP5 (Coupled Model Intercomparison et al., 2018) of the solar energy arriving to the top of the Project) climate models, we quantify the influence of polar Earth atmosphere (short-wave radiation; SW) is absorbed at clouds on the radiative impact of polar sea ice variability. -
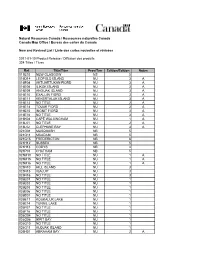
20110110 CMO Maps
Natural Resources Canada / Ressources naturelles Canada Canada Map Office / Bureau des cartes du Canada New and Revised List / Liste des cartes nouvelles et révisées 2011-01-10 Product Release / Diffusion des produits 324 Titles / Titres Ref. Title/Titre Prov/Terr Edition/Édition Notes 011E10 NEW GLASGOW NS 5 016D14 LEOPOLD ISLAND NU 3 A 016E04 AKTIJARTUKAN FIORD NU 3 A 016E06 ILIKOK ISLAND NU 3 A 016E09 ANGIJAK ISLAND NU 3 A 016E10 EXALUIN FIORD NU 3 A 016E11 KEKERTALUK ISLAND NU 3 A 016E13 NO TITLE NU 2 A 016E14 TOUAK FIORD NU 2 A 016E15 INGNIT FIORD NU 3 A 016E16 NO TITLE NU 3 A 016K04 CAPE WALSINGHAM NU 1 A 016L01 NO TITLE NU 2 A 016L02 CLEPHANE BAY NU 3 A 021G01 MUSQUASH NB 5 021G11 MCADAM NB 5 021G15 FREDERICTON NB 6 021H12 SUSSEX NB 5 021H13 CODYS NB 4 021P03 CHATHAM NB 5 025M10 NO TITLE NU 1 A 025M15 NO TITLE NU 1 A 025M16 NO TITLE NU 1 A 025N10 HILL ISLAND NU 3 025N15 IQALUIT NU 3 025N16 NO TITLE NU 3 026E01 NO TITLE NU 1 026E02 NO TITLE NU 1 026E03 NO TITLE NU 1 026E06 NO TITLE NU 1 026E07 NO TITLE NU 1 026E11 KOUKALUK LAKE NU 1 026E14 TUNNIL LAKE NU 1 026F07 NO TITLE NU 1 026F16 NO TITLE NU 1 026G04 NO TITLE NU 1 026G06 IKPIT BAY NU 1 026G10 NO TITLE NU 1 026G11 KUDJAK ISLAND NU 1 026H01 ABRAHAM BAY NU 2 A 026H02 QUEENS CAPE NU 2 A 026H06 SHOMEO POINT NU 2 A 026H07 KUMLIEN FIORD NU 2 A 026H08 UJUKTUK FIORD NU 2 A 026H10 NO TITLE NU 2 A 026H11 IQALUJJUAQ FIORD NU 2 A 026H12 KEKERTEN ISLAND NU 2 A 026H13 KEKERTUKDJUAK ISLAND NU 2 A 026H14 NO TITLE NU 2 A 026I03 KINGNAIT HARBOUR NU 2 A 026I04 PANGNIRTUNG NU 2 A 026I05 MOON PEAK -

Bathymetric Mapping of the North Polar Seas
BATHYMETRIC MAPPING OF THE NORTH POLAR SEAS Report of a Workshop at the Hawaii Mapping Research Group, University of Hawaii, Honolulu HI, USA, October 30-31, 2002 Ron Macnab Geological Survey of Canada (Retired) and Margo Edwards Hawaii Mapping Research Group SCHOOL OF OCEAN AND EARTH SCIENCE AND TECHNOLOGY UNIVERSITY OF HAWAII 1 BATHYMETRIC MAPPING OF THE NORTH POLAR SEAS Report of a Workshop at the Hawaii Mapping Research Group, University of Hawaii, Honolulu HI, USA, October 30-31, 2002 Ron Macnab Geological Survey of Canada (Retired) and Margo Edwards Hawaii Mapping Research Group Cover Figure. Oblique view of new eruption site on the Gakkel Ridge, observed with Seafloor Characterization and Mapping Pods (SCAMP) during the 1999 SCICEX mission. Sidescan observations are draped on a SCAMP-derived terrain model, with depths indicated by color-coded contour lines. Red dots are epicenters of earthquakes detected on the Ridge in 1999. (Data processing and visualization performed by Margo Edwards and Paul Johnson of the Hawaii Mapping Research Group.) This workshop was partially supported through Grant Number N00014-2-02-1-1120, awarded by the United States Office of Naval Research International Field Office. Partial funding was also provided by the International Arctic Science Committee (IASC), the US Polar Research Board, and the University of Hawaii. 2 Table of Contents 1. Introduction...............................................................................................................................5 Ron Macnab (GSC Retired) and Margo Edwards (HMRG) 2. A prototype 1:6 Million map....................................................................................................5 Martin Jakobsson, CCOM/JHC, University of New Hampshire, Durham NH, USA 3. Russian Arctic shelf data..........................................................................................................7 Volodja Glebovsky, VNIIOkeangeologia, St. Petersburg, Russia 4. -

Chapter 7 Arctic Oceanography; the Path of North Atlantic Deep Water
Chapter 7 Arctic oceanography; the path of North Atlantic Deep Water The importance of the Southern Ocean for the formation of the water masses of the world ocean poses the question whether similar conditions are found in the Arctic. We therefore postpone the discussion of the temperate and tropical oceans again and have a look at the oceanography of the Arctic Seas. It does not take much to realize that the impact of the Arctic region on the circulation and water masses of the World Ocean differs substantially from that of the Southern Ocean. The major reason is found in the topography. The Arctic Seas belong to a class of ocean basins known as mediterranean seas (Dietrich et al., 1980). A mediterranean sea is defined as a part of the world ocean which has only limited communication with the major ocean basins (these being the Pacific, Atlantic, and Indian Oceans) and where the circulation is dominated by thermohaline forcing. What this means is that, in contrast to the dynamics of the major ocean basins where most currents are driven by the wind and modified by thermohaline effects, currents in mediterranean seas are driven by temperature and salinity differences (the salinity effect usually dominates) and modified by wind action. The reason for the dominance of thermohaline forcing is the topography: Mediterranean Seas are separated from the major ocean basins by sills, which limit the exchange of deeper waters. Fig. 7.1. Schematic illustration of the circulation in mediterranean seas; (a) with negative precipitation - evaporation balance, (b) with positive precipitation - evaporation balance. -

Elpidia Soyoae, a New Species of Deep-Sea Holothurian (Echinodermata) from the Japan Trench Area
Species Diversity 25: 153–162 Published online 7 August 2020 DOI: 10.12782/specdiv.25.153 Elpidia soyoae, a New Species of Deep-sea Holothurian (Echinodermata) from the Japan Trench Area Akito Ogawa1,2,4, Takami Morita3 and Toshihiko Fujita1,2 1 Graduate School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan E-mail: [email protected] 2 Department of Zoology, National Museum of Nature and Science, 4-1-1 Amakubo, Tsukuba, Ibaraki 305-0005, Japan 3 National Research Institute of Fisheries Science, Japan Fisheries Research and Education Agency, 2-12-4 Fukuura, Kanazawa-ku, Yokohama, Kanagawa 236-8648, Japan 4 Corresponding author (Received 23 October 2019; Accepted 28 May 2020) http://zoobank.org/00B865F7-1923-4F75-9075-14CB51A96782 A new species of holothurian, Elpidia soyoae sp. nov., is described from the Japan Trench area, at depths of 3570– 4145 m. It is distinguished from its congeners in having: four or five paired papillae and unpaired papillae present along entire dorsal radii (four to seven papillae on each radius), with wide separation between second and third paired papillae; maximum length of Elpidia-type ossicles in dorsal body wall exceeds 1000 µm; axis diameter of dorsal Elpidia-type ossicles less than 40 µm; tentacle Elpidia-type ossicles with arched axis and shortened, occasionally completely reduced arms and apophyses. Purple pigmentation spots composed of small purple particles on both dorsal and ventral body wall. This is the second species of Elpidia Théel, 1876 from Japanese abyssal depths. The diagnosis of the genus Elpidia is modified to distin- guish from all other elpidiid genera. -

Plunging Into Our Polar Seas
2021 NOSB Theme Plunging Into Our Polar Seas As the North and South Poles are the two coldest The Earth’s polar regions are perhaps our planet’s climatic regions on Earth, they play a vital role in most unique ecosystems - the Arctic dominated by regulating climate - acting as our planet’s cooling system. The global climate is controlled through a floating sea ice and the Antarctic by ice sheets process called thermohaline circulation. As sea ice on the continent. The vast, isolated expanses of forms at the poles, the remaining salty, dense water snow and ice, and the life which inhabits it, sinks and is replaced by warmer, fresher surface water. have fascinated mankind for ages. Yet much of This water movement creates the deep-ocean currents the Arctic and Antarctic remain unexplored that move cold and warm water around the globe. as they are characterized by extremely Unfortunately, the polar regions are currently at-risk due to cold temperatures, heavy glaciation, and continually increasing levels of anthropogenic carbon dioxide in the atmosphere. Carbon dioxide raises the global temperature extreme variations in daylight hours (24 by trapping heat that would otherwise escape directly into space hours of daylight in the summer and - and the poles are warming at much faster rates than anywhere complete darkness in mid-winter). else on the globe. In the Arctic sea ice cover is declining, as is the Fascination may have been the ratio of thick and thin ice cover. The amount of multi-year ice present impetus for polar research beginning in the Arctic has declined each year since the 1980’s due to warming temperatures, leaving mostly newer, thinner ice on the surface. -

20100930 CMO Maps
Natural Resources Canada / Ressources naturelles Canada Canada Map Office / Bureau des cartes du Canada New and Revised List / Liste des cartes nouvelles et révisées 2010-09-30 Product Release / Diffusion des produits 237 Titles / Titres Ref. Title/Titre Prov/Terr Edition/Édition Notes 001N05 ARGENTIA NL 4 A 001N06 HOLYROOD NL 5 A 001N10 ST JOHN'S NL 9 001N11 HARBOUR GRACE NL 4 002D09 GLOVERTOWN NL 4 A 002D13 GRAND FALLS NL 5 A 002D15 GANDER NL 5 002D16 GAMBO NL 4 012H05 LOMOND NL 4 012H12 GROS MORNE NL 4 016D13 CAPE MERCY NU 3 A 016D14 LEOPOLD ISLAND NU 3 A 016E04 AKTIJARTUKAN FIORD NU 3 A 016E06 ILIKOK ISLAND NU 3 A 016E09 ANGIJAK ISLAND NU 3 A 016E10 EXALUIN FIORD NU 3 A 016K12 MOONSHINE RIDGE NU 3 A 021G03 ST STEPHEN NB 5 021G09 HAMPSTEAD NB 6 021G16 GRAND LAKE NB 7 021H10 ALMA NB/NS 5 021H11 WATERFORD NB 5 021H15 HILLSBOROUGH NB 5 021I10 RICHIBUCTO NB 3 025M11 NO TITLE NU 1 A 025N09 BURTON BAY NU 4 A 025O06 BRUCE ISLAND NU 1 A 025P08 BREVOORT ISLAND NU 1 A 026E08 NO TITLE NU 1 026E09 NO TITLE NU 1 026E10 NO TITLE NU 1 026E15 NO TITLE NU 1 026E16 NO TITLE NU 1 026F01 NO TITLE NU 1 026F02 NO TITLE NU 1 026F03 NO TITLE NU 1 026F04 NO TITLE NU 1 026F05 NO TITLE NU 1 026F06 NO TITLE NU 1 026F08 NO TITLE NU 1 026F09 NO TITLE NU 1 026F10 NO TITLE NU 1 026F11 NO TITLE NU 1 026F12 NO TITLE NU 1 026F13 NO TITLE NU 1 026F14 NO TITLE NU 1 026F15 NO TITLE NU 1 026G01 NO TITLE NU 1 026G02 KANGIGUTSAK ISLAND NU 1 026G03 NO TITLE NU 1 026G05 NO TITLE NU 1 026G07 AUPALUKTUT ISLAND NU 1 026I09 CIRCLE LAKE NU 2 A 026I10 MOUNT FLEMING NU 2 026I16 AVALIKONG -

Canada's Arctic Marine Atlas
Lincoln Sea Hall Basin MARINE ATLAS ARCTIC CANADA’S GREENLAND Ellesmere Island Kane Basin Nares Strait N nd ansen Sou s d Axel n Sve Heiberg rdr a up Island l Ch ann North CANADA’S s el I Pea Water ry Ch a h nnel Massey t Sou Baffin e Amund nd ISR Boundary b Ringnes Bay Ellef Norwegian Coburg Island Grise Fiord a Ringnes Bay Island ARCTIC MARINE z Island EEZ Boundary Prince i Borden ARCTIC l Island Gustaf E Adolf Sea Maclea Jones n Str OCEAN n ait Sound ATLANTIC e Mackenzie Pe Ball nn antyn King Island y S e trait e S u trait it Devon Wel ATLAS Stra OCEAN Q Prince l Island Clyde River Queens in Bylot Patrick Hazen Byam gt Channel o Island Martin n Island Ch tr. Channel an Pond Inlet S Bathurst nel Qikiqtarjuaq liam A Island Eclipse ust Lancaster Sound in Cornwallis Sound Hecla Ch Fitzwil Island and an Griper nel ait Bay r Resolute t Melville Barrow Strait Arctic Bay S et P l Island r i Kel l n e c n e n Somerset Pangnirtung EEZ Boundary a R M'Clure Strait h Island e C g Baffin Island Brodeur y e r r n Peninsula t a P I Cumberland n Peel Sound l e Sound Viscount Stefansson t Melville Island Sound Prince Labrador of Wales Igloolik Prince Sea it Island Charles ra Hadley Bay Banks St s Island le a Island W Hall Beach f Beaufort o M'Clintock Gulf of Iqaluit e c n Frobisher Bay i Channel Resolution r Boothia Boothia Sea P Island Sachs Franklin Peninsula Committee Foxe Harbour Strait Bay Melville Peninsula Basin Kimmirut Taloyoak N UNAT Minto Inlet Victoria SIA VUT Makkovik Ulukhaktok Kugaaruk Foxe Island Hopedale Liverpool Amundsen Victoria King -

Ice Navigation in Canadian Waters
Ice Navigation in Canadian Waters Published by: Icebreaking Program, Maritime Services Canadian Coast Guard Fisheries and Oceans Canada Ottawa, Ontario K1A 0E6 Cat. No. Fs154-31/2012E-PDF ISBN 978-1-100-20610-3 Revised August 2012 ©Minister of Fisheries and Oceans Canada 2012 Important Notice – For Copyright and Permission to Reproduce, please refer to: http://www.dfo-mpo.gc.ca/notices-avis-eng.htm Note : Cette publication est aussi disponible en français. Cover photo: CCGS Henry Larsen in Petermann Fjord, Greenland, by ice island in August 2012. Canadian Coast Guard Ice Navigation in Canadian Waters Record of Amendments RECORD OF AMENDMENTS TO ICE NAVIGATION IN CANADIAN WATERS (2012 VERSION) FROM MONTHLY NOTICES TO MARINERS NOTICES TO INSERTED DATE SUBJECT MARINERS # BY Note: Any inquiries as to the contents of this publication or reports of errors or omissions should be directed to [email protected] Revised August 2012 Page i of 153 Canadian Coast Guard Ice Navigation in Canadian Waters Foreword FOREWORD Ice Navigation in Canadian Waters is published by the Canadian Coast Guard in collaboration with Transport Canada Marine Safety, the Canadian Ice Service of Environment Canada and the Canadian Hydrographic Service of Fisheries and Oceans Canada. The publication is intended to assist ships operating in ice in all Canadian waters, including the Arctic. This document will provide Masters and watchkeeping crew of vessels transiting Canadian ice-covered waters with the necessary understanding of the regulations, shipping support services, hazards and navigation techniques in ice. Chapter 1, Icebreaking and Shipping Support Services, pertains to operational considerations, such as communications and reporting requirements as well as ice advisories and icebreaker support within Canadian waters.