1 Kernel Evolution: from Teosinte to Maize
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Transcript of Flowchart Showing the Uses of Corn
Flowchart Showing the Uses of Corn, 2009 Corn Whole Corn Products Dry Grind Ethanol Fractionated Products Dry-milled Corn Wet-milled Corn Native Starch Cob & Kernel Whole Kernel Products Cob or Stover Alkali Cooked Fermentation Grits & Cones Flour Hominy Feed Germ Oil Meal Steepwater Germ Gluten Feed Gluten Meal Modified Starch Sweeteners Glucose Fructose Fermentation Food baby corn pickled baby corn boiled sweet corn canned corn frozen packaged IOWA DEPARTMENT OF CULTURAL AFFAIRS 600 E. LOCUST ST. DES MOINES, IA 50319 IOWACULTURE.GOV Industrial Decorative items (pod & Indian corn) Food popcorn snack food corn nuts posole canned corn soup mixes canned hominy frozen packaged Feed livestock feed wild animal feed Industrial polishing media furfural (chemical feedstock) liquid spill recovery media dust adsorbent construction board cosmetic powders Food tortilla flours hominy corn chips tortilla chips taco shells sopapillas atoles posole menudo tostados Industrial & Fuel fuel ethanol distillers dried grains with solubles oil for biodiesel Food breakfast cereals fortified foods pinole snack foods maize porridges alkali cooked products breads & bakery products fermented beverages unfermented beverages pet foods corn bread Industrial wallpaper paste floor wax hand soap dusting agents Food bakery products masa flour snack foods baby foods baking mixes batters desserts pie fillings gravies & sauces salad dressings frozen foods meat extenders non-meat extenders thickening agents Industrial fermentation media explosives gypsum wallboard paper -

Corn Has Diverse Uses and Can Be Transformed Into Varied Products
Maize Based Products Compiled and Edited by Dr Shruti Sethi, Principal Scientist & Dr. S. K. Jha, Principal Scientist & Professor Division of Food Science and Postharvest Technology ICAR-Indian Agricultural Research Institute, Pusa New Delhi 110012 Maize is also known as Corn or Makka in Hindi. It is one of the most versatile crops having adaptability under varied agro-climatic conditions. Globally, it is known as queen of cereals due to its highest genetic yield potential among the cereals. In India, Maize is grown throughout the year. It is predominantly a kharif crop with 85 per cent of the area under cultivation in the season. The United States of America (USA) is the largest producer of maize contributing about 36% of the total production. Production of maize ranks third in the country after rice and wheat. About 26 million tonnes corn was produced in 2016-17 from 9.6 Mha area. The country exported 3,70,066.11 MT of maize to the world for the worth of Rs. 1,019.29 crores/ 142.76 USD Millions in 2019-20. Major export destinations included Nepal, Bangladesh Pr, Myanmar, Pakistan Ir, Bhutan The corn kernel has highest energy density (365 kcal/100 g) among the cereals and also contains vitamins namely, vitamin B1 (thiamine), B2 (niacin), B3 (riboflavin), B5 (pantothenic acid) and B6. Although maize kernels contain many macro and micronutrients necessary for human metabolic needs, normal corn is inherently deficient in two essential amino acids, viz lysine and tryptophan. Maize is staple food for human being and quality feed for animals. -

Post-Harvest Operations
MAIZE Post-harvest Operations - Post-harvest Compendium MAIZE: Post-Harvest Operation Organisation:Food and Agriculture Organization of the United Nations (FAO), AGST Author: Danilo Mejía, PhD, AGST. Edited by AGST/FAO: Danilo Mejía, PhD, FAO (Technical) Last reviewed: 15/05/2003 1. Introduction ........................................................................................................................ 2 1.1 Economic and Social Impact. ...................................................................................... 7 1.2 World trade ................................................................................................................ 12 1.3 Maize primary products. ............................................................................................ 15 1.4 Secondary and derived products from maize ............................................................. 21 1.5 Requirements for export and quality assurance. ........................................................ 28 1.6 Consumer preferences. ............................................................................................... 30 1.7 Others. ........................................................................................................................ 32 2. Post-production Operations ......................................................................................... 38 2.1. Pre-harvest operations. .............................................................................................. 38 2.2. Harvesting ................................................................................................................ -

Jeffery's Catering Product Ingredients
JEFFERY’S CATERING PRODUCT INGREDIENTS CEREAL CORN FLAKES BOWL #4265708 – INGREDIENTS: MILLED CORN, SUGAR, SALT, MALT EXTRACT, CORN SYRUP, VITAMIN C (SODIUM ASCORBATE), REDUCED IRON, ZINC (ZINC OXIDE), NIACIN (NIACINAMIDE), VITAMIN A PALMITATE, VITAMIN B6 (PYRIDOXINE HYDROCHLORIDE), VITAMIN D, VITAMIN B2 (RIBOFLAVIN), VITAMIN B1 (THIAMIN MONONITRATE), FOLATE (FOLIC ACID), VITAMIN B12, WHEAT STARCH. BHT TO PRESERVE FRESHNESS. CONTAINS WHEAT SERVING SIZE: 1 BOWL CALORIES 80, TOTAL FAT 0G, CARBOHYDRATE 18G, PROTEIN 2G, SAT. FAT 0G, TRANS FAT 0G KIX WHOLE GRAIN SS BOWL # 1072586 – INGREDIENTS: WHOLE GRAIN CORN, CORN MEAL, SUGAR, CORN BRAN, SALT, BROWN SUGAR SYRUP, TRISODIUM PHOSPHATE. VITAMIN E (MIXED TOCOPHEROLS) ADDED TO PRESERVE FRESHNESS.VITAMINS AND MINERALS: CALCIUM CARBONATE, IRON AND ZINC (MINERAL NUTRIENTS), VITAMIN C (SODIUM ASCORBATE), B VITAMIN (NIACINAMIDE), VITAMIN B6 (PYRIDOXINE HYDROCHLORIDE), VITAMIN B2 (RIBOFLAVIN), VITAMIN B1 (THIAMIN MONONITRATE), VITAMIN A (PALMITATE), B VITAMIN (FOLIC ACID), VITAMIN B12, VITAMIN D3. SERVING SIZE: 1 BOWL PACK, CALORIES 60, TOTAL FAT 0.5G, CARBOHYDRATE 15G, PROTEIN 1G, SUGARS 2G, FIBER 2G RICE CRISPY BOWL # 1265701 – INGREDIENTS: RICE, SUGAR, SALT, HIGH FRUCTOSE CORN SYRUP, MALT EXTRACT, FERRIC ORTHOPHOSPHATE (IRON), VITAMIN C (ASCORBIC ACID), ZINC (ZINC OXIDE), NIACIN (NIACINAMIDE), VITAMIN A PALMITATE, VITAMIN B6 (PYRIDOXINE HYDROCHLORIDE), VITAMIN B2 (RIBOFLAVIN), VITAMIN B1 (THIAMIN HYDROCHLORIDE), VITAMIN D, FOLATE (FOLIC ACID), VITAMIN B12. BHT (TO PRESERVE FRESHNESS). SERVING SIZE: 1 BOWL PACK CALORIES 70, TOTAL FAT 0G, CARBOHYDRATE 16G, PROTEIN 1G, SUGARS 2G, FIBER 0G TOASTY O’S BOWL # 7738776 – INGREDIENTS: WHOLE GRAIN OAT FLOUR (INCLUDES THE OAT BRAN), OAT BRAN, WHEAT STARCH, CONTAINS 2% OR LESS OF: SALT, TRISODIUM PHOSPHATE, CARAMEL COLOR. -

Quality Protein Maize, Specialty and Other Corn Types Production
Quality Protein Maize, Specialty and other Corn types production technology Other than grain, maize is also cultivated for various purposes like quality protein maize and other special purposes known as ‘Specialty Corn’. The various specialty corn types are quality protein maize (QPM), baby corn, sweet corn, pop corn, waxy corn, high oil corn etc. In India, QPM, baby corn and sweet corn are being popularized and cultivated by the large number of farmers. The brief summary of different type of specialty maize is as follows – i. Quality Protein Maize As more than 85 % of the maize is used directly for food and feed, the quality has a great role for food and nutritional security in the country. In this respect, discovery of Opaque-2 (O2) and floury-2 (F2) mutant had opened tremendous possibilities for improvement of protein quality of maize which later led to the development of “Quality Protein Maize (QPM). QPM which is nutritionally superior over the normal maize is the new dynamics to signify its importance not only for food and nutritional security but also for quality feed for poultry, piggery and animal sectors as well. Quality Protein Maize has specific features of having balanced amount of amino acids with high content of lysine and tryptophan and low content of leucine & isoleucine. The balanced proportion of all these essential amino acid in Quality Protein Maize enhances the biological value of protein. The biological value of protein in QPM is just double than that of normal maize protein which is very close to the milk protein as the biological value of milk and QPM proteins are 90 and 80 % respectively. -

Guide to Kashrus – Does It Need a Hechsher (Certification)? (Year-Round, Not Pesach/Passover)
Guide to Kashrus – Does it Need a Hechsher (Certification)? (Year-round, not Pesach/Passover) Edited November 2018 CONTENTS BABY FOOD ................................................................. 1 FRUIT ............................................................................ 6 BAKED/COOKED PRODUCTS ................................. 1 HEALTH AISLE ............................................................ 7 BAKING/COOKING ITEMS ........................................ 1 JELLIES & SPREADS ................................................. 8 BEANS ........................................................................... 2 KITCHEN CLEANING SUPPLIES ............................. 8 BEVERAGES ................................................................ 2 MEAT & POULTRY ..................................................... 9 CONDIMENTS & DRESSINGS ................................. 3 ORAL HYGIENE & MEDICINAL PRODUCTS ........ 9 DAIRY PRODUCTS ..................................................... 4 PET FOOD .................................................................... 9 DESSERTS ................................................................... 4 SPICES AND SEASONINGS..................................... 9 DIPS & SAUCES .......................................................... 5 SIDE DISH ITEMS ..................................................... 10 DISPOSABLE UTENSILS & FOOD WRAPS........... 5 SNACK FOODS ......................................................... 10 EGGS ............................................................................ -

December 10, 2019 | Taj Hotel, Chandigarh
CROP DIVERSIFICATION WITH MAIZE IN PUNJAB SUSTAINABLE AGRICULTURE CONCLAVE DECEMBER 10, 2019 | TAJ HOTEL, CHANDIGARH ( ) FOREWORD 01 FROM THE CHIEF GUEST Sh. J.P.S Bindra, Chief General Manager, NABARD Punjab FROM THE CHAIRMAN'S DESK 02 Mr. Rajinder Gupta, Chairman, FICCI Regional Council-Chandigarh PROGRAM 03 ABOUT THE PROGRAM 04-06 PROGRAM AGENDA KNOWLEDGE BANK 07 PREFACE Why Crop Diversification with Maize in Punjab? 0385-22 Need for Crop Diversification with Maize in Punjab: Way Forward Where Does Punjab Stand on Maize Crop Role of Maize in Animal Nutrition Designing Maize using Novel Techniques for Productivity Enhancement in Punjab Potential of Maize Products & Economic Gains Application of Remote Sensing in Soil Health Studies Farmers Perspective on Maize Cultivation: Few Challenges, Big Opportunities Contamination Control Strategies in Maize Future is Bright for Maize Consumption in Ethanol IN THE NEWS F O R E W O R D | 0 2 FROM THE CHAIRMAN'S DESK Punjab's struggle with groundwater depletion has not remained unknown. It is well-established that paddy-dominant agriculture in Punjab (39.64% of total crop production) has ripped the State of its valuable water resources, so much so that the groundwater level now remains at a critical edge. To address the concern, State-Government as well as State offices of Central bodies have explicitly explored the array of available solutions in various committee meetings and panel discussions, post which crop diversification with less-water intensive crops like maize was accepted as the best suited option in hand. Converting this solution into a reality, NABARD Punjab has been incentivising Punjab farmers for 'shifting from paddy to maize cultivation' under its project, that covered 1000 acres in its first year of implementation itself. -
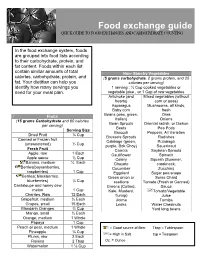
Food Exchange Guide QUICK GUIDE to FOOD EXCHANGES and CARBOHYDRATE COUNTING
Food exchange guide QUICK GUIDE TO FOOD EXCHANGES AND CARBOHYDRATE COUNTING In the food exchange system, foods are grouped into food lists according to their carbohydrate, protein, and fat content. Foods within each list contain similar amounts of total Non- Starchy Vegetables calories, carbohydrate, protein, and (5 grams carbohydrate, 2 grams protein, and 25 fat. Your dietitian can help you calories per serving) identify how many servings you 1 serving : ½ Cup cooked vegetables or need for your meal plan. vegetable juice , or 1 Cup of raw vegetables Artichoke (and Mixed vegetables (without hearts) corn or peas) Asparagus Mushrooms, all kinds, Baby corn fresh Beans (wax, green, Okra Fruits Italian) Onions (15 grams Carbohydrate and 60 calories Bean Sprouts Oriental radish, or Daikon per serving) Beets Pea Pods Serving Size Broccoli Peppers, All Varieties Dried Fruit ¼ Cup Brussels Sprouts Radishes Canned or Frozen fruit Cabbage (green, Rutabaga (unsweetened) ½ Cup purple, Bok Choy) Sauerkraut Fresh Fruit Carrots Soybean Sprouts Apple, raw 1 Each Cauliflower Spinach Apple sauce ½ Cup Celery Squash (Summer, Banana, medium ½ Each Chayote crookneck, Berries(boysenberries, Cucumber Zucchini) raspberries) 1 Cup Eggplant Sugar pea snaps Berries( blackberries, Green onion or Swiss Chard blueberries) ¾ Cup scallions Tomato (Fresh or Canned) Cantaloupe and honey dew Greens (Collard, Sauce melon 1 Cup Kale, Mustard, Tomato/Vegetable Cherries, Raw 12 Each Turnip) Juice Grapefruit, medium ½ Each Jicima Turnips Grapes, small 15 Each Leeks Water Chestnuts Mandarin Oranges ¾ Cup Yard long beans Mango, small ½ Each Orange, medium 1 Whole Papaya 1 Cup Peach or pear, medium 1 Whole = Good source of fiber Tbsp = Tablespoon Pineapple ¾ Cup = High in Salt tsp = Teaspoon Plums, raw 2 Each Raisins 2 Tbsp Oz. -

I Can Tell You About Carbohydrates
Our Journey with Diabetes Si usted desea esta información en español, por favor pídasela a su enfermero o doctor. I can tell you about carbohydrates Children with diabetes should eat the same healthy foods as other children. They do not have to be on a special diet. However, children with type 1 diabetes need to match the amount of insulin to the carbohydrate they will eat. All food is made of different combinations of 3 nutrients: carbohydrates, proteins, and fats. The body uses each of these for different things and digests them in different ways. Carbohydrates (or carbs) are the sugars and starches in food. When eaten, they break down into sugar. Carbohydrates make blood sugar levels go up, whether you have diabetes or not. Insulin lowers the blood sugar levels. In a person who does not have diabetes, the pancreas releases just the right amount of insulin each time food is eaten. This insulin moves the sugar from the blood into the body’s cells, where it is used for energy. In type 1 diabetes, the pancreas no longer makes insulin to take care of the sugar from these foods, so we need to take insulin. In other words, we are doing what the pancreas would have done. The goal is to match the carbs to the insulin as best as we can. Children need carbohydrates to grow and develop. Children with diabetes need carbohydrates, just like they did before getting diabetes. Having type 1 diabetes does not mean your child needs fewer carbs or more carbs. It just means they need the insulin to cover those carbs. -

Enrique Olvera Mexico from Side a Side B the Inside out Pujol Chapter
Enrique Olvera Mexico from Side A Side B the Inside Out Pujol Chapter 7 Un estómago chilango 15 Tradition 67 Product 121 Veracruz Fish Ceviche 121 Roasted Octopus by Juan Villoro 21 Baby Corn with Chicatana Ant, 67 Seepweed Salad and Habanero Pepper Aguachile Coffee, and Costeño Chile Mayonnaise 67 Leek, Bone Marrow, and Ant Larvae 121 Mussel Tostada 12 Circles 23 Bocol Huasteco 67 Corn Truffle, Heirloom Tomatoes, 121 Tamales by Enrique Olvera 25 Puffy Tortilla with Egg, Beans, and Mexican Tarragon 121 Fried Eggs with Bean Salsa and Avocado Leaf Bean Purée, and Grasshopper Salsa 67 Green Mole 121 Octopus, Peanut Sauce, 27 Pumpkin Tamal, 67 Vegetable Mole and Morita Pepper Tostada Sikil Pak, and Jocoque Foam 67 Pickled Mushrooms 155 Burrata in Green Salsa with Quelites 29 Green Salsa Salad 67 Barbecue Eggplant, Isthmus Puree, 155 Squash Blossom Quesadillas 31 Wheat Esquites and Pickled Flowers 155 Scallop, Coconut, and Lime Ceviche 33 Quail, Chorizo, Bean, 67 Purslane Noodles 155 Shell and Mexicola Avocado 67 Parsnip Ice Cream with Amaranth, 155 Divorced Chilaquiles 35 Mexican Creole Hairless Pig, Queso Añejo, and Sweet Tomatillo Salad 155 Vegetable Soup Almond Mole, Tamarind, Cauliflower 67 Black Sapote Sorbet; Kumquat, 189 Mexican Cuttlefish 37 Fried Pork Belly, Smoked Bean Puree, Jasmine Tea, Bergamot, and Vanilla 189 Veracruz Sandwich and Purslane Salad 67 Tonka Bean Ice Cream, Ground Cherry, 189 Aguas frescas 39 Sea Bass al Pastor Café de Olla, and Lime 189 Fish Ceviche Tostada 41 Avocado Cream 67 Hot Chocolate 189 Eggs with Mexican -
Nuestras Famosas Botanas
THE STARTER “Esquites” Freshly shaved roasted corn kernels, limes, mayonnaise, chilli powder and fresh cheese 87 “Guacamole” Mashing ripe avocados and sea salt with a “molcajete” served with tomatoes, onions, lemon juice, coriander 95 Melted Cheese Cheddar cheese, garlic and Pico de Gallo, Served with nachos 78 “Nachos” Tortilla chips “Totopos”, refried beans, baked in oven topped with sour cream, Jalapeno chili and Guacamole NATURAL 165 WITH SHREDDED CHICKEN TINGA 195 WITH CHILLI BEEF 210 Mexican Crudités Cucumbers, carrots, pineapple and baby corn Served with a chilli-mango dip 75 Healthy Cuisine Created using fresh and nutritionally balanced ingredients, dishes contribute to optimal health and wellness Spicy All prices are quoted in Egyptian Pound (LE) and are subject to applicable fees and taxes as well as 12% service charge FROM DE “COMAL” “Quesadillas” A wheat tortilla filled with Cheddar cheese, heated on a “comal” and folded in half WITH CHICKEN 183 WITH BEEF 355 WITH SHRIMP 212 VEGETARIAN 135 “Sincronizada” Wheat tortilla sandwich with refried beans and Cheddar cheese between two flour tortillas served with Guacamole, Pico de Gallo and Jalapeno chili WITH CHICKEN 205 WITH BEEF 135 WITH SHRIMP 315 VEGETARIAN 145 Shrimp Tempura “Taco” Corn tortilla, chipotle mayonnaise, lettuce, red onions and coriander 155 Tacos Basket Soft steamed corn tortilla, mashed potatoes, onions and garlic 57 Healthy Cuisine Created using fresh and nutritionally balanced ingredients, dishes contribute to optimal health and wellness Spicy All prices are -

Manual Chapter - Cuisine (5 January 1993) H
•· I Manual Chapter - Cuisine (5 January 1993) H. cuisine cuisine is used to describe the culinary derivation of a food. H.1 Definition cuisine is characterized by dietary staples and foods typically consumed; specific ingredients in mixed dishes; types of fats, oils, seasonings, and sauces used; food preparation techniques and cooking methods; and dietary patterns. The culinary characteristics of population groups have developed and continue to develop over time. Cuisines have traditional names based primarily on geographic origin. A few cuisine names reflect ethnicity or other factors. Cuisines with several or multiple influences are listed in the hierarchy according to their major influence. Descriptors from this factor should be used primarily for prepared food products (e.g., entrees, desserts, cheeses, breads, sausages, and wines). Descriptors for cuisine should only be used if the cuisine can be easily determined from external evidence such as: the food name; a cuisine indication on a food label; the culinary identification of a restaurant, recipe, or cookbook; or the country of origin of the food, unless another cuisine is indicated. The indexer is not required to make a judgement about cuisine, nor is the indexer required to examine a food to determine its cuisine. Note that some food names have geographic descriptors that do not always identify a cuisine (e.g., Swiss cheese, Brussels sprouts). If in doubt, refer to the foods already indexed to determine whether the food name indicates a specific cuisine. The cuisine of foods may be important in establishing relationships of diet to health and disease. Cuisine provides information about a food from a cultural viewpoint and may assist in assist in more clearly identifying a food.