Key to Marine Arthropod Larvae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Zootaxa 1285: 1–19 (2006) ISSN 1175-5326 (Print Edition) ZOOTAXA 1285 Copyright © 2006 Magnolia Press ISSN 1175-5334 (Online Edition)
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Ghent University Academic Bibliography Zootaxa 1285: 1–19 (2006) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ ZOOTAXA 1285 Copyright © 2006 Magnolia Press ISSN 1175-5334 (online edition) A checklist of the marine Harpacticoida (Copepoda) of the Caribbean Sea EDUARDO SUÁREZ-MORALES1, MARLEEN DE TROCH 2 & FRANK FIERS 3 1El Colegio de la Frontera Sur (ECOSUR), A.P. 424, 77000 Chetumal, Quintana Roo, Mexico; Research Asso- ciate, National Museum of Natural History, Smithsonian Institution, Wahington, D.C. E-mail: [email protected] 2Ghent University, Biology Department, Marine Biology Section, Campus Sterre, Krijgslaan 281–S8, B-9000 Gent, Belgium. E-mail: [email protected] 3Royal Belgian Institute of Natural Sciences, Invertebrate Section, Vautierstraat 29, B-1000, Brussels, Bel- gium. E-mail: [email protected] Abstract Recent surveys on the benthic harpacticoids in the northwestern sector of the Caribbean have called attention to the lack of a list of species of this diverse group in this large tropical basin. A first checklist of the Caribbean harpacticoid copepods is provided herein; it is based on records in the literature and on our own data. Records from the adjacent Bahamas zone were also included. This complete list includes 178 species; the species recorded in the Caribbean and the Bahamas belong to 33 families and 94 genera. Overall, the most speciose family was the Miraciidae (27 species), followed by the Laophontidae (21), Tisbidae (17), and Ameiridae (13). Up to 15 harpacticoid families were represented by one or two species only. -

Emergent and Non-Emergent Species of Harpacticoid Copepods Can Be Recognized Morphologically
MARINE ECOLOGY PROGRESS SERIES Vol. 266: 195–200, 2004 Published January 30 Mar Ecol Prog Ser Emergent and non-emergent species of harpacticoid copepods can be recognized morphologically David Thistle*, Linda Sedlacek Department of Oceanography, Florida State University, Tallahassee, Florida 32306-4320, USA ABSTRACT: Emergence — the active movement of benthic organisms into the water column and back — has consequences for many ecological processes, e.g. benthopelagic coupling. Harpacticoid copepods are conspicuous emergers, but technical challenges have made it difficult to determine which species emerge, impeding the study of the ecology and evolution of the phenomenon. We examined data on harpacticoid emergence from 2 sandy, subtidal sites (~20 m deep) in the northern Gulf of Mexico and found 6 species that always emerged and 2 species that never emerged. An examination of the locomotor appendages revealed that the number of segments in the endopods of pereiopods 2–4 and the number of setae and spines on the distal exopod segments of pereiopods 2–4 can be used to distinguish emergers from non-emergers. We then successfully used these characters to predict the behavior of 3 additional species. Certain morphological differences may therefore allow differentiation of emergers from non-emergers. KEY WORDS: Emergence · Harpacticoid copepods · Continental shelf · Benthopelagic coupling Resale or republication not permitted without written consent of the publisher INTRODUCTION What appear to be emergent harpacticoids have been found in such varied environments as sandy The active movement of individual benthic animals beaches, seagrass meadows, mudflats, coral reefs, and from the seabed into the water column and back, the continental shelf; therefore, harpacticoid emer- often with a diel periodicity, is termed ‘emergence’ gence might be widespread. -

Introduction to Arthropod Groups What Is Entomology?
Entomology 340 Introduction to Arthropod Groups What is Entomology? The study of insects (and their near relatives). Species Diversity PLANTS INSECTS OTHER ANIMALS OTHER ARTHROPODS How many kinds of insects are there in the world? • 1,000,0001,000,000 speciesspecies knownknown Possibly 3,000,000 unidentified species Insects & Relatives 100,000 species in N America 1,000 in a typical backyard Mostly beneficial or harmless Pollination Food for birds and fish Produce honey, wax, shellac, silk Less than 3% are pests Destroy food crops, ornamentals Attack humans and pets Transmit disease Classification of Japanese Beetle Kingdom Animalia Phylum Arthropoda Class Insecta Order Coleoptera Family Scarabaeidae Genus Popillia Species japonica Arthropoda (jointed foot) Arachnida -Spiders, Ticks, Mites, Scorpions Xiphosura -Horseshoe crabs Crustacea -Sowbugs, Pillbugs, Crabs, Shrimp Diplopoda - Millipedes Chilopoda - Centipedes Symphyla - Symphylans Insecta - Insects Shared Characteristics of Phylum Arthropoda - Segmented bodies are arranged into regions, called tagmata (in insects = head, thorax, abdomen). - Paired appendages (e.g., legs, antennae) are jointed. - Posess chitinous exoskeletion that must be shed during growth. - Have bilateral symmetry. - Nervous system is ventral (belly) and the circulatory system is open and dorsal (back). Arthropod Groups Mouthpart characteristics are divided arthropods into two large groups •Chelicerates (Scissors-like) •Mandibulates (Pliers-like) Arthropod Groups Chelicerate Arachnida -Spiders, -

From Ghost and Mud Shrimp
Zootaxa 4365 (3): 251–301 ISSN 1175-5326 (print edition) http://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2017 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4365.3.1 http://zoobank.org/urn:lsid:zoobank.org:pub:C5AC71E8-2F60-448E-B50D-22B61AC11E6A Parasites (Isopoda: Epicaridea and Nematoda) from ghost and mud shrimp (Decapoda: Axiidea and Gebiidea) with descriptions of a new genus and a new species of bopyrid isopod and clarification of Pseudione Kossmann, 1881 CHRISTOPHER B. BOYKO1,4, JASON D. WILLIAMS2 & JEFFREY D. SHIELDS3 1Division of Invertebrate Zoology, American Museum of Natural History, Central Park West @ 79th St., New York, New York 10024, U.S.A. E-mail: [email protected] 2Department of Biology, Hofstra University, Hempstead, New York 11549, U.S.A. E-mail: [email protected] 3Department of Aquatic Health Sciences, Virginia Institute of Marine Science, College of William & Mary, P.O. Box 1346, Gloucester Point, Virginia 23062, U.S.A. E-mail: [email protected] 4Corresponding author Table of contents Abstract . 252 Introduction . 252 Methods and materials . 253 Taxonomy . 253 Isopoda Latreille, 1817 . 253 Bopyroidea Rafinesque, 1815 . 253 Ionidae H. Milne Edwards, 1840. 253 Ione Latreille, 1818 . 253 Ione cornuta Bate, 1864 . 254 Ione thompsoni Richardson, 1904. 255 Ione thoracica (Montagu, 1808) . 256 Bopyridae Rafinesque, 1815 . 260 Pseudioninae Codreanu, 1967 . 260 Acrobelione Bourdon, 1981. 260 Acrobelione halimedae n. sp. 260 Key to females of species of Acrobelione Bourdon, 1981 . 262 Gyge Cornalia & Panceri, 1861. 262 Gyge branchialis Cornalia & Panceri, 1861 . 262 Gyge ovalis (Shiino, 1939) . 264 Ionella Bonnier, 1900 . -

Contributions to the 12Th Conference of the European Wildlife Disease Association (EWDA) August 27Th – 31St, 2016, Berlin
12th Conference of the European Wildlife Disease Association (EWDA), Berlin 2016 Contributions to the 12th Conference of the European Wildlife Disease Association (EWDA) August 27th – 31st, 2016, Berlin, Germany Edited by Anke Schumann, Gudrun Wibbelt, Alex D. Greenwood, Heribert Hofer Organised by Leibniz Institute for Zoo and Wildlife Research (IZW) Alfred-Kowalke-Straße 17 10315 Berlin Germany www.izw-berlin.de and the European Wildlife Disease Association (EWDA) https://sites.google.com/site/ewdawebsite/ & ISBN 978-3-9815637-3-3 12th Conference of the European Wildlife Disease Association (EWDA), Berlin 2016 Published by Leibniz Institute for Zoo and Wildlife Research (IZW) Alfred-Kowalke-Str. 17, 10315 Berlin (Friedrichsfelde) PO Box 700430, 10324 Berlin, Germany Supported by Deutsche Forschungsgemeinschaft (DFG) [German Research Foundation] Kennedyallee 40, 53175 Bonn, Germany Printed on Forest Stewardship Council certified paper All rights reserved, particularly those for translation into other languages. It is not permitted to reproduce any part of this book by photocopy, microfilm, internet or any other means without written permission of the IZW. The use of product names, trade names or other registered entities in this book does not justify the assumption that these can be freely used by everyone. They may represent registered trademarks or other legal entities even if they are not marked as such. Processing of abstracts: Anke Schumann, Gudrun Wibbelt Setting and layout: Anke Schumann, Gudrun Wibbelt Cover: Diego Romero, Steven Seet, Gudrun Wibbelt Word cloud: ©Tagul.com Printing: Spree Druck Berlin GmbH www.spreedruck.de Order: Leibniz Institute for Zoo and Wildlife Research (IZW) Forschungsverbund Berlin e.V. PO Box 700430, 10324 Berlin, Germany [email protected] www.izw-berlin.de 12th Conference of the European Wildlife Disease Association (EWDA), Berlin 2016 CONTENTS Foreword ................................................................................................................. -

Phylum Arthropod Silvia Rondon, and Mary Corp, OSU Extension Entomologist and Agronomist, Respectively Hermiston Research and Extension Center, Hermiston, Oregon
Phylum Arthropod Silvia Rondon, and Mary Corp, OSU Extension Entomologist and Agronomist, respectively Hermiston Research and Extension Center, Hermiston, Oregon Member of the Phyllum Arthropoda can be found in the seas, in fresh water, on land, or even flying freely; a group with amazing differences of structure, and so abundant that all the other animals taken together are less than 1/6 as many as the arthropods. Well-known members of this group are the Kingdom lobsters, crayfish and crabs; scorpions, spiders, mites, ticks, Phylum Phylum Phylum Class the centipedes and millipedes; and last, but not least, the Order most abundant of all, the insects. Family Genus The Phylum Arthropods consist of the following Species classes: arachnids, chilopods, diplopods, crustaceans and hexapods (insects). All arthropods possess: • Exoskeleton. A hard protective covering around the outside of the body (divided by sutures into plates called sclerites). An insect's exoskeleton (integument) serves as a protective covering over the body, but also as a surface for muscle attachment, a water-tight barrier against desiccation, and a sensory interface with the environment. It is a multi-layered structure with four functional regions: epicuticle (top layer), procuticle, epidermis, and basement membrane. • Segmented body • Jointed limbs and jointed mouthparts that allow extensive specialization • Bilateral symmetry, whereby a central line can divide the body Insect molting or removing its into two identical halves, left and right exoesqueleton • Ventral nerve -
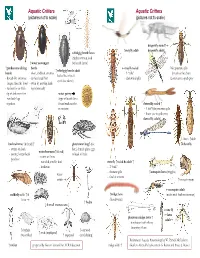
Aquatic Critters Aquatic Critters (Pictures Not to Scale) (Pictures Not to Scale)
Aquatic Critters Aquatic Critters (pictures not to scale) (pictures not to scale) dragonfly naiad↑ ↑ mayfly adult dragonfly adult↓ whirligig beetle larva (fairly common look ↑ water scavenger for beetle larvae) ↑ predaceous diving beetle mayfly naiad No apparent gills ↑ whirligig beetle adult beetle - short, clubbed antenna - 3 “tails” (breathes thru butt) - looks like it has 4 - thread-like antennae - surface head first - abdominal gills Lower jaw to grab prey eyes! (see above) longer than the head - swim by moving hind - surface for air with legs alternately tip of abdomen first water penny -row bklback legs (fbll(type of beetle larva together found under rocks damselfly naiad ↑ in streams - 3 leaf’-like posterior gills - lower jaw to grab prey damselfly adult↓ ←larva ↑adult backswimmer (& head) ↑ giant water bug↑ (toe dobsonfly - swims on back biter) female glues eggs water boatman↑(&head) - pointy, longer beak to back of male - swims on front -predator - rounded, smaller beak stonefly ↑naiad & adult ↑ -herbivore - 2 “tails” - thoracic gills ↑mosquito larva (wiggler) water - find in streams strider ↑mosquito pupa mosquito adult caddisfly adult ↑ & ↑midge larva (males with feather antennae) larva (bloodworm) ↑ hydra ↓ 4 small crustaceans ↓ crane fly ←larva phantom midge larva ↑ adult→ - translucent with silvery bflbuoyancy floats ↑ daphnia ↑ ostracod ↑ scud (amphipod) (water flea) ↑ copepod (seed shrimp) References: Aquatic Entomology by W. Patrick McCafferty ↑ rotifer prepared by Gwen Heistand for ACR Education midge adult ↑ Guide to Microlife by Kenneth G. Rainis and Bruce J. Russel 28 How do Aquatic Critters Get Their Air? Creeks are a lotic (flowing) systems as opposed to lentic (standing, i.e, pond) system. Look for … BREATHING IN AN AQUATIC ENVIRONMENT 1. -

Invert3 2 115 135 Abiahy at All.PM6
Invertebrate Zoology, 2006, 3(2): 115-135 INVERTEBRATE ZOOLOGY, 2006 published online 06.01.2007 accepted for publication 21.11.2006 Redescription of Limnoithona tetraspina Zhang et Li, 1976 (Copepoda, Cyclopoida) with a discussion of character states shared with the Oithonidae and older cyclopoids Bernardo Barroso do Abiahy\ Carlos Eduardo Falavigna da Rocha^, Frank D. Ferrari^ ' Avenida Manuel Hipolito do Rego 1270/ap. 09, 11.600-000 Sao Sebastiao, SP, Brasd ^ Universidade de Sao Paulo, Instituto de Biociencias, Departamento de Zoologia, Rua do Matdo, travessa 14, No. 321, 05508-900 Sao Paulo, Brazd 'Department of Invertebrate Zoology, MRC-534, National Museum of Natural History, Smithso- nian Institution, 4210 Silver Hill Rd., Suitland, MD 20746 U.S.A. ABSTRACT: Limnoithona tetraspina Zhang et Li, 1976 is redescribed, and the morpho- logy of the cephalosome, rostral area, oral appendages, legs 1-6 andurosome of adult males and females is illustrated. Morphological features separating L. tetraspina from its only congener,Z.5/«e«5;5, include: a more pronounced rostrum; 1 seta more on the proximal lobe of the basis of the maxillule; 1 seta more on the endopod of the maxillule; middle endopodal segment of swimming legs 2-A with 1 seta more; proximal and distal seta of the middle endopodal segment of swimming leg 4 with a flange; exopod of leg 5 with a proximal lateral seta; male cephalosome ventrally with pores with cilia. A rounded projection between labrum and rostrum is a shared derived state for both species of Limnoithona. Derived morphological features of the remaining species of Oithonidae, which are not shared with L. -

Fishery Bulletin/U S Dept of Commerce National Oceanic
LARVAL DEVELOPMENT OF THE CUBAN STONE CRAB, MENlPPE NODlFRONS (BRACHYURA, XANTHIDAE), UNDER LABORATORY CONDITIONS WITH NOTES ON THE STATUS OF THE FAMILY MENIPPIDAE1 LumInA E. SCO,I'02 ABSTRACT The complete larval development of the Cuban stone crab, Menippc nodifrons, is described and illustrated. Larvae reared in the laboratory passed through a prezoeal, five and uncommonly six zoeal, and one megalopal stage. At 30" C the megalopal stage was attained in 16-17 clays, at 20" C, 28-37 days. The six zoeal stages of M. nodifrons are compared with those of its sympatric congener M. mcrc<'!laria and with the first zoeal stage of the Indo-Pacific species M. rumphii. Larvae ofthe genus Menippe may be distinguished from other xanthid larvae by a combination of morphological features, including lilltennal development (exopodite at least three-fourths the length ofprotopoditel, lack of setae on the basal segment of the second maxillipL'<!al endopodite, and number oflarval stages (Menippe, 5 or 6; most other xanthids, 4). Using Lebour's criteria (emphasizing antenna! development and number of zoeal stages) to determine the primitive or advancL'<! status of decapod larvae, the genus Menippe is more closely related to the phylogentically primitive family Cancriclae than to most of the Xanthidae. The possible reestablishment of the family Menippidae is discussed in view of new larval evidence. The Cuban stone crab, MCllippe llodij'rolls tions of West Africa (Capart 1951; Monod 1956), a Stimpson 1859, is a medium-sized xanthid crab survey of stomatopod and decapod crustaceans of closely allied to the common commercial stone Portuguese Guiana (Vilela ]951), and an ecologi crab, MCllippc I/lcrcenaria (Say 18] 8). -

Visceral and Cutaneous Larva Migrans PAUL C
Visceral and Cutaneous Larva Migrans PAUL C. BEAVER, Ph.D. AMONG ANIMALS in general there is a In the development of our concepts of larva II. wide variety of parasitic infections in migrans there have been four major steps. The which larval stages migrate through and some¬ first, of course, was the discovery by Kirby- times later reside in the tissues of the host with¬ Smith and his associates some 30 years ago of out developing into fully mature adults. When nematode larvae in the skin of patients with such parasites are found in human hosts, the creeping eruption in Jacksonville, Fla. (6). infection may be referred to as larva migrans This was followed immediately by experi¬ although definition of this term is becoming mental proof by numerous workers that the increasingly difficult. The organisms impli¬ larvae of A. braziliense readily penetrate the cated in infections of this type include certain human skin and produce severe, typical creep¬ species of arthropods, flatworms, and nema¬ ing eruption. todes, but more especially the nematodes. From a practical point of view these demon¬ As generally used, the term larva migrans strations were perhaps too conclusive in that refers particularly to the migration of dog and they encouraged the impression that A. brazil¬ cat hookworm larvae in the human skin (cu¬ iense was the only cause of creeping eruption, taneous larva migrans or creeping eruption) and detracted from equally conclusive demon¬ and the migration of dog and cat ascarids in strations that other species of nematode larvae the viscera (visceral larva migrans). In a still have the ability to produce similarly the pro¬ more restricted sense, the terms cutaneous larva gressive linear lesions characteristic of creep¬ migrans and visceral larva migrans are some¬ ing eruption. -

Remarkable Convergent Evolution in Specialized Parasitic Thecostraca (Crustacea)
Remarkable convergent evolution in specialized parasitic Thecostraca (Crustacea) Pérez-Losada, Marcos; Høeg, Jens Thorvald; Crandall, Keith A Published in: BMC Biology DOI: 10.1186/1741-7007-7-15 Publication date: 2009 Document version Publisher's PDF, also known as Version of record Citation for published version (APA): Pérez-Losada, M., Høeg, J. T., & Crandall, K. A. (2009). Remarkable convergent evolution in specialized parasitic Thecostraca (Crustacea). BMC Biology, 7(15), 1-12. https://doi.org/10.1186/1741-7007-7-15 Download date: 25. Sep. 2021 BMC Biology BioMed Central Research article Open Access Remarkable convergent evolution in specialized parasitic Thecostraca (Crustacea) Marcos Pérez-Losada*1, JensTHøeg2 and Keith A Crandall3 Address: 1CIBIO, Centro de Investigação em Biodiversidade e Recursos Genéticos, Universidade do Porto, Campus Agrário de Vairão, Portugal, 2Comparative Zoology, Department of Biology, University of Copenhagen, Copenhagen, Denmark and 3Department of Biology and Monte L Bean Life Science Museum, Brigham Young University, Provo, Utah, USA Email: Marcos Pérez-Losada* - [email protected]; Jens T Høeg - [email protected]; Keith A Crandall - [email protected] * Corresponding author Published: 17 April 2009 Received: 10 December 2008 Accepted: 17 April 2009 BMC Biology 2009, 7:15 doi:10.1186/1741-7007-7-15 This article is available from: http://www.biomedcentral.com/1741-7007/7/15 © 2009 Pérez-Losada et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. -

A Possible 150 Million Years Old Cirripede Crustacean Nauplius and the Phenomenon of Giant Larvae
Contributions to Zoology, 86 (3) 213-227 (2017) A possible 150 million years old cirripede crustacean nauplius and the phenomenon of giant larvae Christina Nagler1, 4, Jens T. Høeg2, Carolin Haug1, 3, Joachim T. Haug1, 3 1 Department of Biology, Ludwig-Maximilians-Universität München, Großhaderner Straße 2, 82152 Planegg- Martinsried, Germany 2 Department of Biology, University of Copenhagen, Universitetsparken 15, 2100 Copenhagen, Denmark 3 GeoBio-Center, Ludwig-Maximilians-Universität München, Richard-Wagner-Straße 10, 80333 Munich, Germany 4 E-mail: [email protected] Key words: nauplius, metamorphosis, palaeo-evo-devo, Cirripedia, Solnhofen lithographic limestones Abstract The possible function of giant larvae ................................ 222 Interpretation of the present case ....................................... 223 The larval phase of metazoans can be interpreted as a discrete Acknowledgements ....................................................................... 223 post-embryonic period. Larvae have been usually considered to References ...................................................................................... 223 be small, yet some metazoans possess unusually large larvae, or giant larvae. Here, we report a possible case of such a giant larva from the Upper Jurassic Solnhofen Lithographic limestones (150 Introduction million years old, southern Germany), most likely representing an immature cirripede crustacean (barnacles and their relatives). The single specimen was documented with up-to-date